Abstract
Birds are well known for occupying diverse feeding niches, and for having evolved diverse beak morphologies associated with dietary specialization. Birds that feed on hard seeds typically possess beaks that are both deep and wide, presumably because of selection for fracture avoidance, as suggested by prior studies. It follows then that birds that eat seeds of different size and hardness should vary in one or more aspects of beak morphology, including the histological organization of the rhamphotheca, the cellular interface that binds the rhamphotheca to the bone, and the organization of trabeculae in the beak. To explore this expectation we here investigate tissue organization in the rhamphotheca of the Java finch, a large granivorous bird, and describe interspecific differences in the trabecular organization of the beak across 11 species of Darwin's finches. We identify specializations in multiple layers of the horny beak, with the dermis anchored to the bone by Sharpey's fibers in those regions that are subjected to high stresses during biting. Moreover, the rhamphotheca is characterized by a tight dermo-epidermal junction through interdigitations of these two tissues. Herbst corpuscles are observed in high density in the dermis of the lateral aspect of the beak as observed in other birds. Finally, the trabecular organization of the beak in Darwin's finches appears most variable in regions involved most in food manipulation, with the density of trabeculae in the beak generally mirroring loading regimes imposed by different feeding habits and beak use in this clade.
Keywords: beak anatomy, birds, Darwin's finches, Herbst corpuscles, histology, Padda oryzivora, trabecular structure and organization
Introduction
Birds are an ecologically diverse and species-rich group of terrestrial vertebrates that have radiated extensively into a variety of habitats and trophic niches (Mayaud, 1950; McLelland, 1979; Raikow & Bledsoe, 2000). Bird beaks are equally diverse, and in many species the diversity in beak size and shape appears to be correlated with feeding habits (Newton & Gadow, 1896; Bowman, 1961; Pimm & Pimm, 1982; Grant, 1999). Amongst birds, specializations for feeding on hard seeds are common, and many species that feed on hard seeds typically have beaks that are both deep and broad, suggesting that the shape of the beak plays an important role in allowing birds to crack hard seeds. Yet, the ability to crack hard seeds depends primarily on birds' ability to generate high bite forces, via jaw muscles. Consequently, the beak can be thought of as a structure shaped by selection for fracture avoidance as suggested by studies on the mechanical resistance of the beak to loading (Herrel et al. 2005b, 2010; Soons et al. 2010, 2012a,b). Indeed, the evolution of high bite force capacity has gone hand in hand with the evolution of large beak size independently in many radiations of birds specialized in the cracking of hard seeds (Thomson, 1923; Mayaud & Grassé, 1950; van der Meij & Bout, 2004; Herrel et al. 2005a,b). Yet, other selective pressures also act on the beak (e.g. Herrel et al. 2009), and the final structure and shape likely reflect a compromise between all of the selective pressures involved.
One of the best studied examples of an adaptive radiation in feeding form and function is Darwin's finches of the Galápagos and Cocos Islands. Darwin's finches are traditionally classified into three functional groups according to their feeding habits and beak morphology (Bowman, 1961; Grant, 1986): first, the crushers with deep, broad beaks (ground finches: Geospiza magnirostris, G. fortis, G. fuliginosa, G. conirostris); second, the probers with long, narrow beaks (Certhidea olivacea, Pinaroloxias inornata, Cactospiza pallida, Ca. heliobates, Geospiza scandens); and third, the tip-biters with curved upper and lower beaks (tree finches: Camarhynchus parvulus, C. pauper, C. psittacula). Geospiza difficilis and Platyspiza crassirostris are more difficult to assign to any one of these groups. Geospiza difficilis has a beak that is deep at its base but is also fairly straight, and thus falls between the first and second groups. Platyspiza crassirostris combines characteristics from the second and third groups, and uses the entire length of its beak for biting (Bowman, 1961). Surprisingly, and despite the fact that these finches are some of the best studied cases of adaptive differentiation of morphology in relation to diet, virtually nothing is known about fine-scale morphological adaptations and functional specializations associated with the evolution of their ability to crack hard seeds (but see Bowman, 1961; Herrel et al. 2005a,b, 2009, 2010; Soons et al. 2010). The protected status and restrictions associated with obtaining living or even fresh, dead specimens of Darwin's finches have complicated functional morphological studies.
To explore the hypothesis that beak shape may have evolved in order to avoid fracture in seed-eating birds (Bowman, 1961; Herrel et al. 2005a,b; Soons et al. 2010), researchers have used mechanical models, including finite element models, to predict how different beak morphologies in seed-eating birds should be able to withstand reaction forces generated on the beak during biting (Soons et al. 2010; Rayfield, 2011). Although these models offer a good first approximation of how beaks may respond functionally to loads imposed by seed-crushing or pecking, current models are hampered by two major limitations: a lack of data on how the keratinous rhamphotheca (horny beak) contributes to beak strength (but see Soons et al. 2012a,b); and a lack of data concerning the organization of the bony beak's internal trabecular structure. Realistic models, representative of actual feeding behavior in vivo, will require attention to the rhamphotheca, the cellular interface between the horny and bony beak, and the organization of the trabeculae. Moreover, striking variation in the structure and dimensions of the rhamphotheca relative to the underlying bone can be observed in different species of seed-cracking birds (personal observation), consistent with a potentially important role of the keratinous rhamphotheca in stress dissipation during biting (see also Soons et al. 2012b).
Yet, the histological organization of the rhamphotheca, the bony beak and the cellular interface between these two, remains, to our knowledge, poorly known (Lucas & Stettenheim, 1972; Stettenheim, 1972; Spearman, 1973; Stettenheim, 2000; Van Hemert et al. 2012). These studies show that the rhamphotheca is a thick, modified integument that covers the underlying bone. It is hard and heavily cornified in most birds. The epidermis is thick and composed of beta-keratin-producing cells. The dermis, by contrast, is thin and binds the keratinized epidermis to the bone (Spearman, 1973; Stettenheim, 2000). The organization of the trabecular structure of the bony beak has been described for only a few species, including the toucan, the hornbill (Seki et al. 2005) and the crow (Bock, 1966).
Here we explore fine-scale structure and organization of the rhamphotheca, the bony beak and the cellular interface between the two, with particular attention to how this structure and its organization may potentially enable birds to withstand the strains imposed on the beak by seed crushing. We predict distinct differences in tissue organization in those beak regions that endure the highest strains (see Herrel et al. 2010; Soons et al. 2010), and also expect variation in the trabecular organization of the bony beak among the different species of Darwin's finches mirroring variation in how these birds use their beaks (Bowman, 1961). We begin with a detailed description of the histological and structural organization of the rhamphotheca, the bony beak and the cellular interface in the Java finch (Padda oryzivora), a passeriform bird that has been suggested as a suitable model for understanding adaptations to seed cracking (Genbrugge et al. 2011). We then describe, in the Java finch as well as Darwin's finches, the variation in the trabecular structure of the bony beak.
Materials and methods
Specimens
Eight specimens of the Java finch (Padda oryzivora) were used in this study. The Padda oryzivora specimens were obtained from commercial suppliers and killed by a veterinarian of the Faculty of Veterinary Medicine at Ghent University. Six specimens were preserved in a 10% aqueous formaldehyde solution for 4 weeks. One specimen was preserved in Bouin fixative for 4 weeks. Small parts of the beak of the eighth specimen were transferred to a Karnovsky medium (2% paraformaldehyde, 2.5% glutaraldehyde and 0.5% CaCl2 in 0.134 m sodium cacodylate buffer) immediately after killing the specimen. These latter samples were stored in the refrigerator (4 °C) for 1 day.
Data on the trabecular organization of the beak were obtained for 22 specimens of Darwin's finches. Eleven specimens of six species (Camarhynchus parvulus: 2; Certhidea olivacea: 2; Geospiza fortis: 2; G. fuliginosa: 2; G. scandens: 2; and Platyspiza crassirostris: 1) were road-killed birds collected during February–March of 2005 and 2006 on Santa Cruz Island, Galapagos. A stretch of road of approximately 5 km was walked continuously every day between sunrise and 13:00 hours, and all road-killed birds that showed no obvious external damage to the head were collected. Specimens were preserved in a 10% aqueous formaldehyde solution for 24 h, rinsed and transferred to a 70% aqueous ethanol solution. These specimens were collected under a salvage permit from the Galápagos National Park Service. The other 11 Darwin's finch specimens were obtained from the collections of the Museum of Comparative Zoology (Harvard University) and the California Academy of Sciences [Cactospiza pallida (MCZ65744 and CAS ORN 86881), Camarhynchus psittacula (MCZ65738 and CAS ORN 42348), Geospiza difficilis (MCZ39828 and CAS ORN 86586), G. magnirostris (MCZ112397 and CAS ORN 86316), Pineraloxias inornata (MCZ157930 and CAS ORN 86959) and Platyspiza crassirostris (MCZ134639)].
Histological sections
For all specimens of Java finches, histological cross-sections throughout the beak were made by embedding either parts of the beak, a whole beak, or the head. Several embedding media were tested as sectioning of heavily cornified and keratinized tissues proved to be challenging. The embedding media tested were Technovit 7100, Paraffin, Epon and Spurr. For the first three embedding media, specimens were first rinsed with tap water, decalcified with Osteomoll (Merck Cat. No. 1.01736), rinsed again with tap water, dehydrated in a graded (30, 50, 70 and 96%) ethanol solution and transferred to Technovit 7100 (Heraeus Kulzer Wehrheim, Germany), Paraffin (Merk, 9025) or Epon (Fluka, 45359). The small parts of the beak fixed with Karnovsky medium were rinsed with 0.134 m sodium cacodylate buffer for 8 h, decalcified with Osteomoll and rinsed again with the 0.134 m sodium cacodylate buffer. Post-fixation took place overnight in reduced osmium, a mixture of 1 mL OsO4 (4%), 3 mL Na cacodylate (0.134 m) and 66 mg K3Fe(CN)6. After rinsing with double-distilled water, the parts were dehydrated in 50, 70, 96% and absolute ethanol, to which CuSO4 bars were added to remove any remaining water. The specimens were then transferred to Spurr (no 1969).
Histological sections of the specimens embedded in Technovit 7100 were made on a POLYCUT Leica SM2500 microtome equipped with a wolfram carbide knife. The Paraffin-, Epon- and Spurr-embedded specimens were cut with a MICROM HM360 microtome equipped with disposable microtome blades (Superlab; Paraffin) and diamond knives (Epon and Spurr). All sections were cut at a thickness of 2 μm, stained with toluidin blue, mounted with a xylene-based mounting medium and covered.
The histological sections were examined using an Olympus SZX9 stereomicroscope, on which a ColorView 8 digital camera was mounted.
CT-scanning and 3D-reconstruction
All salvaged road-killed specimens were scanned at the UGCT scanning facility at Ghent University (http://www.ugct.ugent.be). Reconstruction of the tomographic projection data was done using the in-house developed Octopus-package (Vlassenbroeck et al. 2007). The specimens obtained from the Museum of Comparative Zoology were scanned at the Harvard CNS facility. Reconstruction of the tomographic projections was done using CTPro (Metris). Voxel sizes ranged from 25.26 μm for the smaller species to 45.75 μm for Geospiza magnirostris.
The CT-data were loaded into Amira 5.2.2 (64-bit version; Computer Systems Mercury) where the data were first reoriented along the x-, y- and z-axes so that all specimens are oriented along the same axes. The bony beak structures were then segmented semi-automatically based on gray-scale values of the voxels, with manual corrections to remove noise. Surface and volume rendering were also performed in Amira 5.2.2. The trabecular organization was described for the posterior third of the beak anterior to the nares in all specimens.
Results
Epidermis
The epidermis is a thick, stratified squamous epithelium made up of multiple layers of cells. Its thickness varies depending on the location on the beak. Three regions can be distinguished: the dorsal and lateral surface; the lateral sharp edge (tomial edge) of the beak; and the horny palate.
The epidermis at the dorsal and lateral surface has more or less the same organization, comprising a stratum basale, stratum spinosum, stratum transitivum and stratum corneum (Fig. 1A–F). The stratum basale consists of a single layer of columnar cells, between which interdigitations (Fig. 1A,D,F) of the dermis can be found. Several cells show projections into the dermis. The stratum spinosum (Fig. 1A,D,E) consists of four–six layers of cells that are characterized by the clearly visible spinae (Fig. 1E) of adjacent cells. The subsequent stratum transitivum (Fig. 1A–D) consists of multiple cell layers with cells that are getting flatter as they move towards the outer surface. The outer layer of the epidermis is the keratinous stratum corneum (Fig. 1A–D) with very flat and dead cells without a nucleus. They lie next to and on top of each other like thin filaments that have been compressed (Fig. 1B). This stratum corneum is thicker than the other three layers together and broadens towards the tomial edge (Fig. 1G). Moving from the caudal part of the beak to its tip, the living part largely maintains its thickness, although the cells of the stratum spinosum and transitivum are somewhat more compressed at the level of the nares. The cells of the stratum transitivum and spinosum are orientated parallel with the surface of the rhamphotheca (Fig. 1K) instead of in line with the cells of the stratum basale. This becomes clearer towards the tip of the beak. The lifeless stratum corneum is relatively thicker at the tip of the beak. The tip of the outer beak is made up of only cells of the stratum corneum.
Fig. 1.
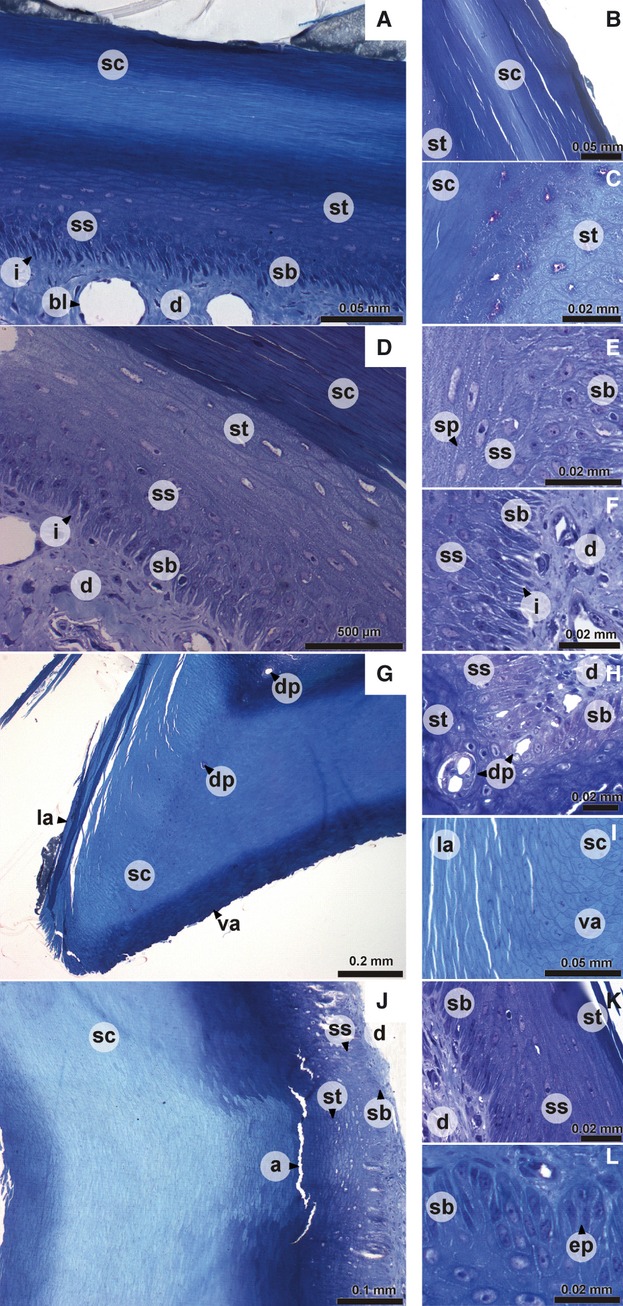
Digital pictures of histological sections of the epidermis of the upper beak of Padda oryzivora. (A) dorsal aspect of the upper beak; (B, C, E, F, K) detail of lateral aspect of the upper beak; (D) lateral aspect of the upper beak; (G) tomial edge; (H, I) detail of tomial edge; (J) ventral aspect of the upper beak; (L) detail of ventral aspect of the upper beak. a, artifact; bl, blood vessel; d, dermis; dp, dermal projection in the epidermis; ep, epidermal papilla; i, interdigitations; la, lateral aspect of the upper beak; sb, stratum basale; sc, stratum corneum; sp, spinae; ss, stratum spinosum; st, stratum transitivum; va, ventral aspect of the upper beak.
The tomial edges (Fig. 1G) are characterized by a very thick epidermal layer, which consists almost completely of keratinized and cornified cells of the stratum corneum. The cells of the stratum corneum are round except at the dorsal and lateral side of the tomial edge. At those points the cells change their shape and become flat cells typical of the lateral aspect of the beak (Fig. 1I). In the center of the stratum corneum of the tomial edge, round packets of uncornified cells can be found (Figs 1G,H and 2G–I). These are probably cells from the stratum basale that surround projections of the dermis into the tomial edge.
Fig. 2.
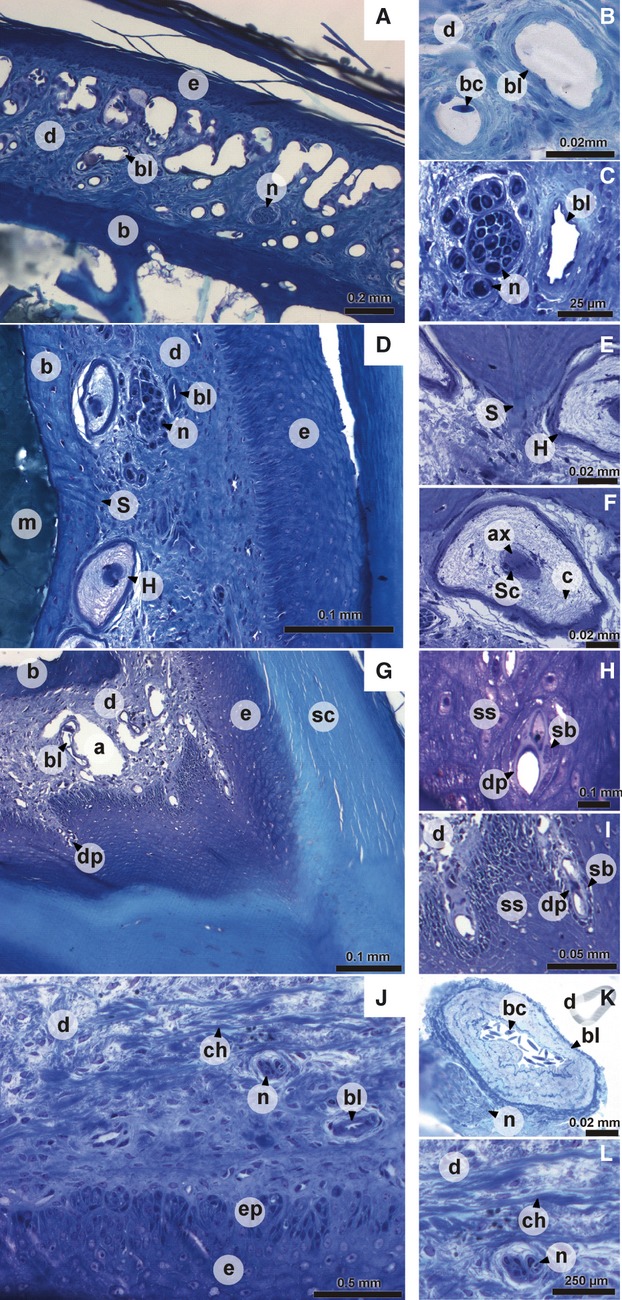
Digital pictures of histological sections of the dermis of the upper beak. (A) dorsal aspect of the upper beak; (B) detail of dorsal aspect of the upper beak; (D) lateral aspect of the upper beak; (C, E, F) detail of lateral aspect of the upper beak; (G) tomial edge; (H, I) detail of tomial edge; (J) ventral aspect of the upper beak; (K, L) detail of ventral aspect of the upper beak. a, artifact; ax, axon; b, bone; bc, blood cell; bl, blood vessel; c, collagen fibers; ch, horizontally oriented collagen bundle; d, dermis; dp, dermal projection in the epidermis; e, epidermis; ep, epidermal papilla; H, Herbst corpuscle; m, bone marrow; n, nerve bundle; S, Sharpey's fiber; sb, stratum basale; sc, stratum corneum; Sc, nucleus of Schwann cell; ss, stratum spinosum.
The epidermis of the horny palate (Fig. 1J) is very thick (two–three times the thickness of the lateral and dorsal surface) and characterized by a well-developed relief with ridges and grooves. The strata basale, spinosum and transitivum in this region have the same thickness as in the lateral and dorsal aspect of the beak. However, the thickness of the stratum corneum of the horny palate is much greater. The cells of the stratum basale are broader than those at the lateral aspect of the beak and are organized in epidermal papillae (Fig. 1L). These dermal papillae are larger at both the base and the tip of the beak. Sometimes a lamina basalis is distinguishable as a darker line between the epidermal cells and the dermis. The appearance of the cells of the stratum corneum changes from the base of the beak to its tip, with cells at the caudal-most point of the beak being very flat. Just anterior the nares, the cells of the stratum corneum are rounder, even at the surface. Going more rostrally, the cells again become more flattened. The grooves and ridges are visible only in the stratum corneum. At some places the organization of the horny palate is also visible in the stratum transitivum as an accumulation of cells at the ridges or a decrease in the number of cells in the grooves (Fig. 1J).
Dermis
The dermis consists of a single layer of dense irregular connective tissue in which blood vessels, mechanoreceptors and nerves can be found. The presence of these elements depends on the region of the beak dermis considered. Four regions can be distinguished: the dorsal aspect, the lateral aspect, the tomial edge and the palate. At the dorsal face of the upper beak (Fig. 2A), the dermis contains numerous large blood vessels (Fig. 2A,B), often running through the entire thickness of the dermis. The dermis in this area also contains a few small nerve bundles (Fig. 2A). The large blood vessels become much smaller when moving more laterally through the beak. Additionally, when going from caudal to rostral the blood vessels become smaller and less numerous. The blood vessels found at the lateral aspect of the beak are generally smaller, less abundant and lie on the epidermic side (Fig. 2C,D). The dermis of the lateral aspect of the beak (Fig. 2D) is characterized by mechanoreceptors together with a larger number of nerve bundles, which have a larger diameter then those of the dorsal aspect of the beak. The mechanoreceptors are Herbst corpuscles that are visible as large corpuscles with a central axon surrounded by nuclei of Schwann cells and a concentric network of collagen fibers (Fig. 2D,F). The nerve bundles and Herbst corpuscles are situated close to the bone (Fig. 2D,C). The Herbst corpuscles can be found at the entire lateral aspect of the beak (see also Van Hemert et al. 2012), but are absent from the tip in front of the bone. At the level of the tomial edge, projections of the dermis run into the epidermis (Figs 1G,H and 2G–I). Possibly, these projections are connected to the small round packets with uncornified cells in the stratum corneum of the tomial edge, described above. However, this cannot be verified based on our histological sections. The dermis of the palate (Fig. 2J) is thinner, and Herbst corpuscles, nerves and blood vessels are scarce. Only medially, several Herbst corpuscles, large bundles of nerves, and large arteries (Fig. 2K) and veins can be observed.
In addition to the presence of sensory and vascular elements, the dermis of the beak is characterized by distinct, dense bundles of collagen fibers. These bundles are very abundant near the base of the beak (at the level of and just anterior to the nares) on both the lateral and dorsal aspect of the beak. The collagen bundles become less abundant and their thickness diminishes more rostrally in the beak. At the lateral and dorsal aspect of the base of the beak, these bundles lie in close contact with the bone and regularly penetrate it as Sharpey's fibers (Fig. 2D,E). These penetrations are mostly associated with small protrusions of the bone. Most of the bundles are oriented dorsally away from the bone. In the dermis of the palate of the beak, the collagen fibers are thinner and oriented more horizontally (Fig. 2L).
Bone
The praemaxillary bone is situated in the center of the beak. It consists of a thick outer bony shell filled with numerous bony, pillar-shaped trabeculae, in a foam-like structure. On its outer surface, the bone shows small protrusions in which collagen fibers penetrate. These protrusions are mostly found in the region just anterior to the nares and especially on the lateral aspect of the bony beak. The spaces between the trabeculae are occupied by adipose tissue. The trabeculae also surround several canals through the bone in which blood vessels and nerve bundles run.
The organization of the internal trabeculae and their thickness differs from species to species. In Padda oryzivora (Fig. 3) the upper beak is filled with numerous, fine trabeculae just anterior to the nares (Fig. 3D). Additionally, the tip of the beak is filled with slender trabeculae (Fig. 3B). In the middle portion of the beak, only the lateral sides of the beak show small trabeculae (Fig. 3C,D). The center is an open space with only a couple of larger and thicker vertically oriented trabeculae present.
Fig. 3.
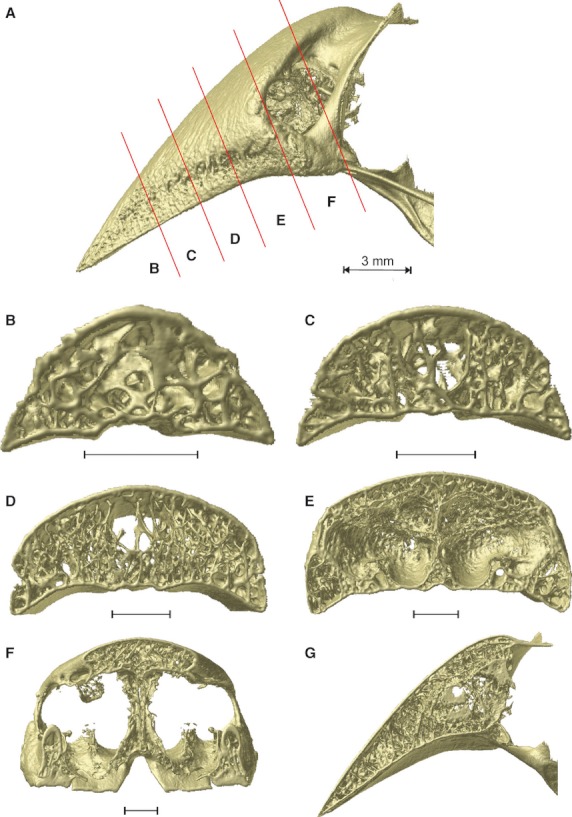
Organization of the trabeculae inside the upper beak bone of Padda oryzivora. (A) Lateral view with the position of the cross-sections indicated by the red lines; (B–F) caudal view of the cross-sections through the upper beak; (G) midsagittal cross-section through the upper beak. Scale bars below the cross-sections represent 1 mm.
Based on the organization and structure of the trabeculae in the upper beak, just anterior to the nares, the Darwin's finches can be divided into four groups largely corresponding to previously defined functional groups (Bowman, 1961; Figs 4 and 5): in the first group a medial zone is observed where trabeculae are concentrated, and comprises the base crushing Geospiza magnirostris, G. fortis, G. fuliginosa; the second group is characterized by two more lateral zones with more intense trabeculation (left and right side of the beak) and a large cavity in the center of the middle third of the beak, and is composed of the species with probing beaks including Geospiza scandens, G. difficilis, Pinaroloxias inornata, Certhidea olivacea; the third group is characterized by thin and seemingly unorganized trabeculae with the entire tip of the beak filled by small trabeculae (this third group comprises the tip-biting species Camarhynchus psittacula, C. parvulus, Cactospiza pallida); the fourth group represents a mix between the morphologies of Groups I and III, and is composed of the vegetarian finch, Platyspiza crassirostris.
Fig. 4.
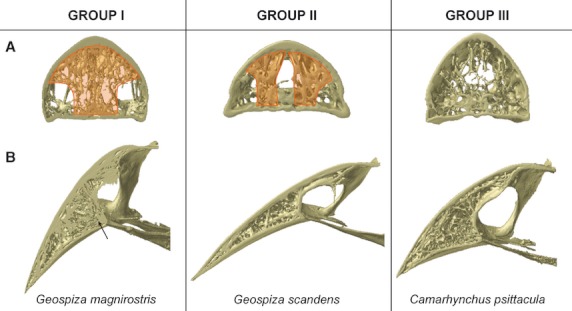
Organization of the trabeculae into three groups, showing: (A) the region just anterior to the nares (cross-section D in Fig. 3) with an indication of the zones of concentrated trabeculation in orange; (B) the midsagittal plane. Note the diverging trabeculae at the point where the os palatinum fuses with the upper beak in Geospiza magnirostris, indicated with an arrow.
Fig. 5.
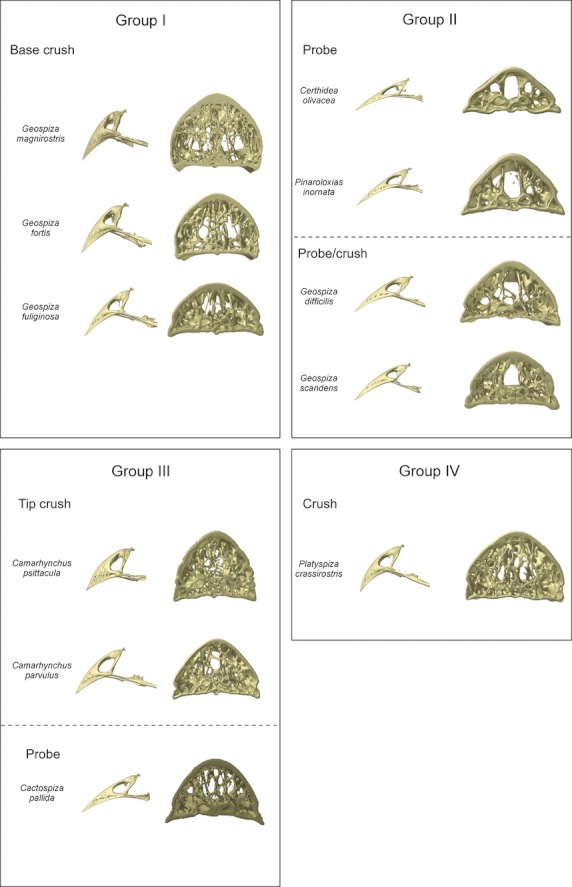
The beaks of the Darwin's finches, demonstrating the variation in beak shape and corresponding variation in trabecular organization. The trabecular organization of the region just anterior to the nares is shown (cross-section D in Fig. 3). The classification of the different species into groups is based on the trabecular anatomy. For each group the functional groupings from Bowman (1961) are also represented. Note that although in the section just anterior to the nares Platyspiza crassirostris resembles the base-crushing morphology of Group I, near the tip of the beak it resembles the morphology of Group III more.
For the first group, concentration of trabeculae in this medial zone is broadest and densest in Geospiza magnirostris, and occupies almost the entire width of the bony beak. The trabeculae in Geospiza fortis tend to fuse with one another, thereby forming a kind of central bony plate. In Geospiza fuliginosa, only a few large trabeculae are situated medially in the beak, rendering it the least ‘typical’ of this group. The other two-thirds of the beak of Geospiza fuliginosa are characterized by a central open space. Only in the outermost lateral corners of the beak and the very tip, can trabeculae be found. This central open space is smaller in Geospiza fortis and is almost absent in G. magnirostris, where nearly the entire beak is filled with trabeculae. Interestingly, in this group the trabeculae within the mediosagittal plane diverge in the region anterior to the fusion of the os palatinum with the os praemaxillare (Fig. 4B).
In the second group, the lateral zones of denser trabeculation, just anterior to the nares, range from being composed of several small trabeculae in Geospiza scandens to one thick trabecula on each side as in Certhidea olivacea. The tip of the beak in these species is filled with trabeculae of which the abundance diminishes going from Geospiza scandens to Certhidea olivacea (in the above-mentioned sequence). Central in the beak a hollow space is situated, which stretches to the dorsal surface of the bony shell, such that only the ventral side and the lateral corners show small trabeculae.
The third group comprises species of which the beaks are completely filled with seemingly unorganized trabeculae just anterior to the nares. The trabeculae are numerous and fine in Camarhynchus psittacula, but larger and less numerous in Cactospiza pallida. The tips of these beaks are also filled with numerous small trabeculae. The middle third of the beak is hollow centrally, with only small trabeculae on the dorsal side and the lateral corners of the beak being present. In Cactospiza pallida this central cavity is largest as it reaches the dorsal side of the beak, although a couple of large trabeculae do cross this space.
The organization of the trabeculae in the beak of Platyspiza crassirostris, the sole representative of Group IV, is unique. It resembles that of Geospiza magnirostris at the point just anterior to the nares, but resembles that of the Camarhynchus species in the organization of the trabeculae in the tip, suggesting that the beak in this species is able to withstand both tip and base loading. Moreover, this illustrates how the trabecular organization across species represents a continuum rather than discrete groups.
Discussion
Beak tissue organization and mechanical stress resistance
Our results accord closely with previously published data (Lucas & Stettenheim, 1972; Sawyer et al. 1986; Bragulla & Homberger, 2009; Van Hemert et al. 2012) in suggesting that the upper beak contains four distinct layers: first, the dead, strongly keratinized outer layer (stratum corneum); second, the living rest of the epidermis (strata transitivum, stratum spinosum = stratum intermedium in Lucas & Stettenheim, 1972 and Sawyer et al. 1986; and stratum basale); third, the dermis; and fourth, the bone. In Padda oryzivora, each layer has specific characteristics that are particularly interesting in the context of potential adaptations to the dissipation of mechanical stress. The flake-like cells of the thick stratum corneum probably form a strong unit as the cytoplasm of these cells is filled with coarse bundles of keratin filaments (consisting of the hard avian β-keratins) and the intercellular matrix is filled with cementing substances (Bloom & Fawcett, 1994; Sawyer et al. 2000; Alibardi, 2009). This organization of hard elements (such as calcium Spearman, 1973; Bragulla & Homberger, 2009 and pigments, Alibardi, 2010) gives the stratum corneum its resistance to abrasion (Bonser & Witter, 1993; Seki et al. 2005). The cells of the underlying cell layers of the strata transitivum, spinosum and basale contain keratin filaments in their cytoplasm, which may allow these cells to withstand mechanical stress, maintain their structural integrity, ensure mechanical resilience and provide protection against variations in hydrostatic pressure (Bragulla & Homberger, 2009). In addition to the strengthening resulting from the specific material properties, at the cellular level, the tissue organization shows similar adaptations. Indeed, on the dorsal and lateral aspect of the beak of Padda oryzivora the cells of the stratum basale interdigitate with the dermis. The cells of the epidermis of the horny palate are also connected with the dermis, although here the cells of the stratum basale are organized in epidermal papillae. In this way, the epidermis and dermis form a tight dermo-epidermal junction. Furthermore, the dermis also shows a strong interaction with the bone as large, dense bundles of collagen fibers make direct contact with the bone of the beak. Several bundles penetrate deep into the bone and can be recognized as Sharpey's fibers (Francillon-Vieillot et al. 1990), anchoring the dermis to the bone. The abundance of the collagen fibers in the dermis in Padda oryzivora likely gives it visco-elastic properties with limited extension capacities, as collagen fibers are only able to compress but not to stretch (Wainwright et al. 1976). The point to which the dermis can be stretched likely depends on the orientation and length of the collagen fibers.
The observed cell orientation in the epidermis of Padda oryzivora may also reflect adaptations to differing levels of mechanical stress. As seed-eating birds crack seeds unilaterally, in the groove between the tomial edge and the lateral rim of the palate (van der Meij & Bout, 2006), the rhamphotheca is expected to experience a torque around the bony core, which should in turn impose shear stress at the interface between the bone and the epidermal cells. More specifically, because the forces induced by cracking a seed are oriented perpendicular to the horny palate at the place where the seed is cracked (van der Meij & Bout, 2006), shear forces can be expected to act at the lateral aspect of the beak. By contrast, the ventral aspect of the beak is expected to have to cope with compressive stress. Our data show that the epidermal cells of the lateral aspect are flat and positioned with their long axis in line with the direction of shear. In contrast, cells of the horny palate are more rounded, making them likely better suited to cope with compressive stresses. Moreover, as mentioned above, the different elements and layers of the beak are firmly anchored to one another. In the dermis at the lateral face of the beak a good anchoring of the rhamphotheca and underlying cellular interface to the bone will be important in resisting the expected shear. It is thus not surprising that there is an abundance of collagen fibers of the dermis at the lateral and dorsal aspect of the beak penetrating into the periosteum and dispersing into the bone. This is in contrast to the horizontally oriented collagen fibers visible at the ventral aspect of the beak, which only occasionally penetrate the periosteum. The finite element models of Soons et al. (2010, 2012a,b) suggest that the stresses in the upper beak induced by the unilateral cracking of a seed at the base of the beak are highest at the dorsal and lateral side of the beak at the level of the nares and lowest at the tip of the beak. These data are in accordance with our observations of the organization of the rhamphotheca, the bony beak and the cellular interface.
The high density of Herbst corpuscles in the dermis is striking. Previously it has been suggested that their function is related to touch, pressure or vibrational stimuli (Mayaud & Grassé, 1950; Portmann, 1950; Stettenheim et al. 1972), or with detecting acceleration components associated with mechanical stimuli (Malinovsky & Pac, 1990). Yet, the exact function of the Herbst corpuscles remains unknown to this date. The frequent occurrence of these corpuscles in the dermis of Padda oryzivora and the Black-capped Chickadee (Van Hemert et al. 2012), especially on the lateral aspect of the beak, suggests to us that they may be part of a feedback system that could allow birds to detect stress in the dermis. Such a feedback system has been suggested for mammals, where periodontal receptors encode information on the orientation, magnitude, rate and position of loads applied to the teeth (Ross et al. 2007; Herrel et al. 2008). As teeth are absent in birds, other receptors are probably responsible for feedback. These corpuscles seem to be less abundant in the beak of the chickens described by Lucas & Stettenheim (1972), but can be very abundant at the tips of the beaks of other birds, including sandpipers (Piersma et al. 1998; Nebel et al. 2005) and Ibises (Cunningham et al. 2010), where they are thought to function in prey detection using remote touch. In these species the corpuscles are always found close to the bone or even in small pits in the bone, but at the tip of the beak, rather than at the lateral aspect of the beak as observed for Padda oryzivora and the Black-capped Chickadee (Van Hemert et al. 2012). Although the Herbst corpuscules may play an important role in providing sensory feedback, it remains puzzling that they are absent from areas of the beak known to be under high stress during biting. As such they may have alternate or additional roles in the beaks of seed-cracking birds.
Trabecular organization of the bony beak
Consistent with our predictions, the organization of the trabeculae varies among species in a manner that appears to coincide more or less with variation in their feeding habits. The first group, containing Geospiza magnirostris, G. fortis and G. fuliginosa, typically crush seeds at the base of the beak (Bowman, 1961). The presence of a medial zone with denser concentration of trabeculae at the base of the beak is thus not surprising and may strengthen the beak. Moreover, the diverging trabeculae anterior to the fusion of the os palatinum with the os praemaxillare may play an important role in strengthening the beak as this region is subjected to large tensile forces (bite forces generated by the pterygoid and retractor palatini muscles are transferred to the beak through the os palatinum during seed cracking; Bowman, 1961; Herrel et al. 2010; Soons et al. 2010; Genbrugge et al. 2011). However, it is also noticeable that the smallest ground finch, G. fuliginosa, displays a morphology that is almost intermediate between that of Groups I and II. Given that this species predominantly cracks soft seeds, it is not surprising that the trabecular organization of its beak shows some resemblance to that of G. scandens or G. difficilis, species with probing beak morphologies. Yet, it diverges from the latter species by the presence of a medial zone with dense trabeculae at the base of the beak.
Representatives of the second group, containing Geospiza scandens, G. difficilis, Pinaroloxias inornata and Certhidea olivacea, mostly use their beak to probe flowers, foliage, fruits and woody tissues (Bowman, 1961; Grant, 1986). Here, the trabeculae are concentrated laterally at the base of the beak and in the tip, while the middle third of the beak is for the largest part hollow. In Geospiza scandens and G. difficilis the lateral zones of denser trabeculation are broader and denser than in the other members of this group. This can probably be related to the fact that these finches use their beak for probing as well as crushing of soft seeds (Bowman, 1961; Grant, 1986). The members of the third group, Camarhynchus psittacula, C. parvulus, Cactospiza pallida, are known to use the tip of their beaks to manipulate fruits and insects, rip bark from trees, or handle tools such as needles and small twigs (Bowman, 1961; Grant, 1986). The trabeculae are seemingly randomly organized at the base of the beak but their tip is completely filled with trabeculae, which may be related to their feeding habits involving predominantly the tip of the beak. The sole representative of the fourth group, Platyspiza crassirostris, shows a morphology combining characteristics from Groups I and III with reinforcements at the base and at the tip of the beak. This corresponds well with the fact that this species uses both the tip and the base of its beak to crush. In general, our results suggest that the regions used most prominently during food manipulation are most variable across species. This is, however, not surprising as the density of trabeculae is often highest in those regions enduring the highest strains (Meyers et al. 2008).
The organization of the trabeculae in the species studied here differs notably from that described for the toucan (Ramphastidae) and the hornbill (Bucerotidae; Seki et al. 2005; Meyers et al. 2008). Inside the bony shell of the beak of the latter species, trabeculae are observed but remain seemingly unorganized and the center of the bony core is hollow. Although a hollow space can also be observed in the species studied here, it is smaller and almost completely lacking in Geospiza magnirostris and Platyspiza crassirostris. In the toucan and hornbill this hollow cavity is thought to function to make the beak lighter (Seki et al. 2005). Given that these birds do not crack seeds, yet transport food by inertial mechanisms, the mechanical constraints on beak function are entirely different and are reflected in a different trabecular organization of the beak. Although trabeculae appear to play an important functional role in strengthening the beak, this remains to be tested explicitly. Given the multitude of selective pressures and constraints operating on beak design (Herrel et al. 2009), the final structure and shape of the beak in Darwin's finches and other birds most likely reflects a compromise phenotype.
Acknowledgments
The authors would like to thank two anonymous referees for their helpful and constructive comments on earlier versions of the paper. Fieldwork was coordinated through the Charles Darwin Research Station and the Galápagos National Park Service. The authors thank Eric Hilton, Sarah Huber and Bieke Vanhooydonck for their assistance in the field and for helping collect road-killed specimens. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the National Science Foundation under NSF award no. ECS-0335765. CNS is part of the Faculty of Arts and Sciences at Harvard University. This work was supported by NSF grant IBN-0347291 to J.P., by an interdisciplinary research grant of the special research fund of the University of Antwerp to P.A., J.D., A.G. and A.H., and by a PHC Tournesol collaborative grant to D.A. and A.H. The Special Research Fund of the Ghent University (BOF) is acknowledged for the doctoral grant to L.B. and the support to UGCT.
Authors' contributions
Acquisition of material: Annelies Genbrugge, Jeffrey Podos, Anthony Herrel; Histological sectioning: Barbara De Kegel; CT-scanning: Loes Brabant, Luc Van Hoorebeke, Anthony Herrel; Drafting of the manuscript: Anthony Herrel, Annelies Genbrugge; Help with methods: Dominique Adriaens, Peter Aerts, Joris Dirckx; Critical revision of the manuscript: all authors.
References
- Alibardi L. Embryonic keratinization in vertebrates in relation to land colonization. Acta Zool. 2009;90:1–17. [Google Scholar]
- Alibardi L. Histology, ultrastructure, and pigmentation in the horny scales of growing crocodilians. Acta Zool. 2010;92:187–200. [Google Scholar]
- Bloom W, Fawcett DW. A Textbook of Histology. 12th edn. New York: Chapman & Hall; 1994. [Google Scholar]
- Bock WJ. An approach to the functional analysis of bill shape. Auk. 1966;113:10–51. [Google Scholar]
- Bonser RHC, Witter MS. Indentation hardness of the bill keratin of the European starling. The Cooper. 1993;95:736–738. [Google Scholar]
- Bowman RI. Morphological differentiation and adaptation in the Galapagos finches. Univ Calif Publ Zool. 1961;58:1–302. [Google Scholar]
- Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–559. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SJ, Alley MR, Castro I, et al. Bill morphology of ibises suggests a remote-tactile sensory system for prey detection. Auk. 2010;127:308–316. [Google Scholar]
- Francillon-Vieillot H, de Buffrénil V, Castanet J. Microstructure and mineralization of vertebrate skeletal tissue. In: Carter JG, et al., editors. Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. Vol. 1. New York: Van Nostrand Reinhold; 1990. pp. 441–530. [Google Scholar]
- Genbrugge A, Herrel A, Boone M, et al. The head of the finch: a detailed analysis of the feeding apparatus in two species of finches (Geospiza fortis and Padda oryzivora. J Anat. 2011;219:676–695. doi: 10.1111/j.1469-7580.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PR. Ecology and Evolution of Darwin's finches. Princeton: Princeton University Press; 1986. [Google Scholar]
- Grant PR. Ecology and Evolution of Darwin's Finches. 2nd edn. Princeton: Princeton University Press; 1999. [Google Scholar]
- Herrel A, Podos J, Huber SK, et al. Bite performance and morphology in a population of Darwin's finches: implications for the evolution of beak shape. Funct Ecol. 2005a;19:43–48. [Google Scholar]
- Herrel A, Podos J, Huber SK, et al. Evolution of bite force in Darwin's finches: a key role for head width. J Evol Biol. 2005b;18:669–675. doi: 10.1111/j.1420-9101.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- Herrel A, Schaerlaeken V, Ross CF, et al. Electromyography and the evolution of motor control: limitations and insights. Integr Comp Biol. 2008;48:261–271. doi: 10.1093/icb/icn025. [DOI] [PubMed] [Google Scholar]
- Herrel A, Podos J, Vanhooydonck B, et al. Force-velocity trade-off in Darwin's finch jaw function: a biomechanical basis for ecological speciation? Funct Ecol. 2009;23:119–125. [Google Scholar]
- Herrel A, Soons J, Aerts P, et al. Adaptation and function of Darwin's finch beaks: divergence by feeding type and sex. Emu. 2010;110:39–47. [Google Scholar]
- Lucas AM, Stettenheim PR. Avian Anatomy – Integument – Part II. Washington DC: US Dept. Agric; 1972. Agricultural Handbook 362. [Google Scholar]
- Malinovsky L, Pac L. Ultrastructure of Herbst corpuscle from beak skin of the pigeon. Zeitschrift Mikroscop Anat Forsch. 1990;94:292–304. [PubMed] [Google Scholar]
- Mayaud N. Tégument et phanères. In: Grassé P-P, editor. Traité de Zoologie: Anatomie, Systématique, Biologie – Tome XV Oiseaux. Paris: Masson et Cie Éditeurs, Libraires de l' Académie de Médicine; 1950. pp. 4–77. [Google Scholar]
- McLelland J. 3 Digestive system. In: King AS, McLelland J, editors. Form and Function in Birds. Vol. 1. London: Academic Press; 1979. pp. 69–92. [Google Scholar]
- van der Meij MAA, Bout RG. Scaling of jaw muscle size and maximal bite force in finches. J Exp Biol. 2004;207:2745–2753. doi: 10.1242/jeb.01091. [DOI] [PubMed] [Google Scholar]
- van der Meij MAA, Bout RG. Seed husking time and maximal bite force in finches. J Exp Biol. 2006;209:3329–3335. doi: 10.1242/jeb.02379. [DOI] [PubMed] [Google Scholar]
- Meyers MA, Chen PY, Lin AYM, et al. Biological materials: structure and mechanical properties. Prog Mater Sci. 2008;53:1–206. [Google Scholar]
- Nebel S, Jackson DL, Elner RW. Functional association of bill morphology and foraging behaviour in calidrid sandpipers. Anim Biol. 2005;55:235–243. [Google Scholar]
- Newton A, Gadow H. A Dictionary of Birds. London: Adam and Charles Black; 1896. [Google Scholar]
- Piersma T, van Aelst R, Kurk K, et al. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proc R Soc Lond B. 1998;265:1377–1383. [Google Scholar]
- Pimm SL, Pimm JW. Resource use, competition, and resource availability in hawaiian honeycreepers. Ecology. 1982;63:1468–1480. [Google Scholar]
- Portmann A. Les organes des sens. In: Grassé P-P, editor. Traité de Zoologie: Anatomie, Systématique, Biologie – Tome XV Oiseaux. Paris: Masson et Cie Éditeurs, Libraires de l' Académie de Médicine; 1950. pp. 204–220. [Google Scholar]
- Raikow RJ, Bledsoe AH. Phylogeny and evolution of the Passerine birds. Bioscience. 2000;50:487–499. [Google Scholar]
- Rayfield EJ. Strain in the ostrich mandible during simulated pecking and validation of specimen-specific finite element models. J Anat. 2011;218:47–58. doi: 10.1111/j.1469-7580.2010.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CF, Eckhardt A, Herrel A, et al. Modulation of intra-oral processing in mammals and lepidosaurs. Integr Comp Biol. 2007;47:118–136. doi: 10.1093/icb/icm044. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Kapp LW, O'Guin WM. The skin of the birds: epidermis, dermis and appendages. In: Bereiter-Hahn J, Matoltsy AG, Richars KS, editors. Biology of the Integument 2 Vertebrates. Berlin: Springer; 1986. pp. 374–408. [Google Scholar]
- Sawyer RH, Glenn T, French JO, et al. The expression of beta (β) keratins in the epidermal appendages of reptiles and birds. Am Zool. 2000;40:530–539. [Google Scholar]
- Seki Y, Schneider MS, Meyers MA. Structure and mechanical behavior of a toucan beak. Acta Mater. 2005;53:5281–5296. [Google Scholar]
- Soons J, Herrel A, Genbrugge A, et al. Mechanical stress, fracture risk and beak evolution in Darwin's ground finches (Geospiza) Phil Trans R Soc Lond B Biol Sci. 2010;365:1093–1098. doi: 10.1098/rstb.2009.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons J, Herrel A, Aerts P, et al. Determination and validation of the elastic moduli of small and complex biological samples: bone and keratin in bird beaks. J R Soc Interface. 2012a;9:1381–1388. doi: 10.1098/rsif.2011.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons J, Herrel A, Genbrugge A, et al. Multi layered bird beaks: a finite-element approach towards the role of keratin in stress dissipation. J R Soc Interface. 2012b;9:1787–1796. doi: 10.1098/rsif.2011.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman RIC. Biological Structure and Function 3: The Integument. Cambridge, MA: Cambridge University Press; 1973. [Google Scholar]
- Stettenheim PR. The integumentary morphology of modern birds – an overview. Am Zool. 2000;40:461–477. [Google Scholar]
- Stettenheim PR. 1. The integument of birds. In: Farner DS, King JR, Parkers KC, editors. Avian Biology II. New York: Academic Press; 1972. pp. 1–63. [Google Scholar]
- Thomson JA. The Biology of Birds. London: Sidgwick and Jackson, LTD; 1923. [Google Scholar]
- Van Hemert C, Handel CM, Blake JE, et al. Microanatomy of passerine hard cornified tissues: beak and claw structure of the Black-capped Chickadee (Poecile atricapilus. J Morphol. 2012;273:226–240. doi: 10.1002/jmor.11023. [DOI] [PubMed] [Google Scholar]
- Vlassenbroeck J, Dierick M, Masschaele B, et al. Software tools for quantification of X-ray microtomography at the UGCT. Nucl Instrum Methods Phys Res A. 2007;580:442–445. [Google Scholar]
- Wainwright SA, Biggs WD, Currey JD, et al. Mechanical Design in Organisms. London: Edward Arnold; 1976. [Google Scholar]


