Figure 1 .
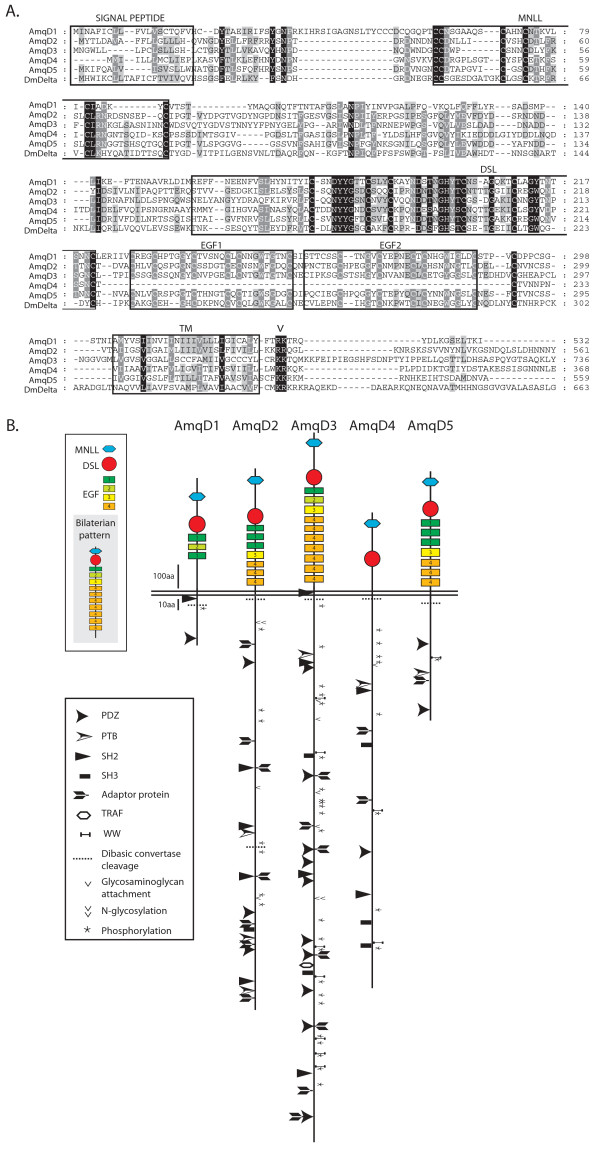
Molecular characteristics of Amphimedon queenslandica Deltas. (A) Alignment of the extracellular regions of A. queenslandica and Drosophila Deltas. All sequences possess a signal peptide and transmembrane (TM) domain, a Delta/Serrate/Lag (DSL) domain, epidermal growth factor (EGF) repeats (except AmqD4) and the conserved pattern of cysteine residues characteristic of the MNLL region. A series of basic residues (V) lies downstream of the TM domain, possibly representing a nuclear localization sequence. The region between EGF2 and the TM domain is omitted. Dashes indicate gaps, residues are shaded according to the level of conservation at each position: 100%, black; 80%, dark grey; 60%, light grey. (B) The diversification of the A. queenslandica Delta ligands is reflected in the number and organization of their extracellular EGF domains, and the distribution and identity of predicted interaction sites in their intracellular tails. EGF repeat identities and bilaterian consensus pattern follow [18]. Potential sites of protein binding, cleavage, phosphorylation and glycosyl modification based on [19]. PTB, phosphotyrosine-binding domain; SH2, Src homology 2; SH3, Src homology 3; TRAF, tumor necrosis factor receptor-associated factor.