Abstract
Background
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear receptors superfamily and are transcription factors activated by specific ligands. Liver X receptors (LXR) belong to the nuclear hormone receptors and have been shown to play an important role in cholesterol homeostasis. From the previous screening of several medicinal plants for potential partial PPARγ agonists, the extracts of Cornus alternifolia were found to exhibit promising bioactivity. In this paper, we report the isolation and structural elucidation of four new compounds and their potential as ligands for PPAR.
Methods
The new compounds were extracted from the leaves of Cornus alternifolia and fractionated by high-performance liquid chromatography. Their structures were elucidated on the basis of spectroscopic evidence and analysis of their hydrolysis products.
Results
Three new iridoid glycosides including an iridolactone, alternosides A-C (1–3), a new megastigmane glycoside, cornalternoside (4) and 10 known compounds, were obtained from the leaves of Cornus alternifolia. Kaempferol-3-O-β-glucopyranoside (5) exhibited potent agonistic activities for PPARα, PPARγ and LXR with EC50 values of 0.62, 3.0 and 1.8 μ M, respectively.
Conclusions
We isolated four new and ten known compounds from Cornus alternifolia, and one known compound showed agonistic activities for PPARα, PPARγ and LXR.
General significance
Compound 1 is the first example of a naturally occurring iridoid glycoside containing a β-glucopyranoside moiety at C-6.
Keywords: Peroxisome proliferator-activated receptors, Liver X receptors, Cornus alternifolia, Iridoid glycosides
1. Introduction
Type II diabetes is a complex, metabolic disorder characterized by hyperglycemia and subsequent chronic complications leading to renal failure, blindness and coronary artery disease. Hyperglycemia in type II diabetes is caused by increased insulin resistance and impaired insulin secretion from the pancreas [1]. The conventional approach to treat type II diabetes focuses on the control of blood glucose levels in order to reduce the incidence of the microvascular and macrovascular complications associated with high levels of blood glucose. Liver X receptors (LXRs) are members of a superfamily of nuclear hormone receptors represented by two subtypes, LXRα and LXRβ. These receptors are differentially expressed and have been shown to play a role in cholesterol homeostasis [2]. Activation of LXRs induces reverse cholesterol transport and increases high-density lipoprotein cholesterol [3]. On the other hand, the peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily of ligand-dependent transcription factors with three isoforms named α, γ and δ. These receptors play a pivotal role in regulating the expression of a large number of genes involved in lipid metabolism and energy balance. PPARα is highly expressed in metabolically active tissues such as the liver, heart and muscle. Activation of PPARα decreases the serum triglyceride level and increases the HDL-c level. PPARγ is expressed predominantly in adipose tissue. Activation of PPARγ increases the insulin sensitivity, promotes the differentiation of lipocytes and retards the occurrence of complications [4,5]. The currently available PPAR agonists aimed at diabetes are known as thiazolidinediones or “glitazones”. These include pioglitazone (brand name Actos) and rosiglitazone (Avandia). These drugs are known to increase the sensitivity of the body’s tissues to the action of insulin. Researchers now recognize that the thiazolidinediones exert this effect by binding to and activating PPARγ. Furthermore, these drugs may inhibit certain proteins in the blood vessel walls called chemokines, which attract inflammatory cells and thus promote atherosclerosis. Researchers have also come to realize that certain drugs called fibrates may work to lower levels of triglycerides (a blood fat) and raise levels of high-density lipoprotein (HDL, or “good”) cholesterol in part by activating PPAR-alpha [6].
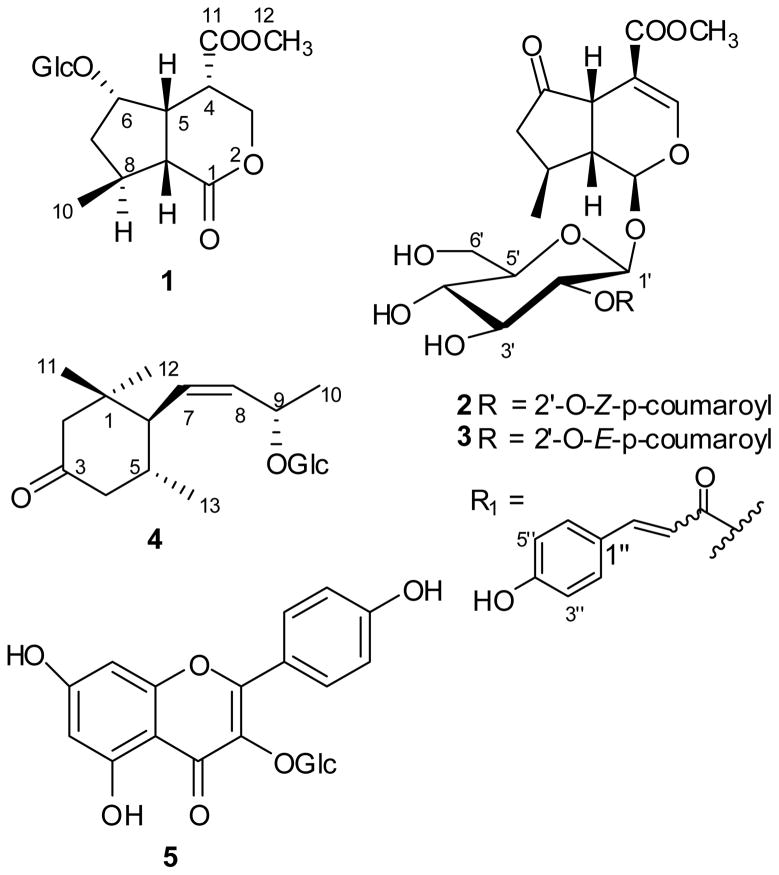
Cornus alternifolia L. f. (Cornaceae) is a tree widely distributed in the northern hemisphere including eastern Asia and eastern and northern parts of the United States [7]. C. alternifolia is widely grown as an ornamental plant throughout the United States for its characteristic brilliant, colorful, and attractive flowers and fruits. Extracts of the plant have been used traditionally in Chinese herbal medicine as tonics, analgesic and diuretic drugs [8]. Previous phytochemical investigations have revealed that the major chemical constituents of this plant are anthocyanins [8,9] which impart bright colors to fruits and vegetables and are responsible for antioxidant, anti-inflammatory [10], anti-cancer [11] and anti-diabetic activities [12]. An investigation of natural products for discovery of nuclear receptor activators we found that an extract of C. alternifolia acted as an agonistic towards PPAR and LXR receptors. The aim of the present study was to explore the structurally unique and biologically active compounds from this plant. Here we report the isolation and structure elucidation of new iridoid glycosides (1–3), named alternosides A-C, and a new megastigmane glycoside (4), named cornalternoside (Fig. 1). Alternoside A (1) represents the first example of naturally occurring iridoid glycoside with a β-glucopyranoside moiety at C-6. The LXR and PPAR agonistic activities of these compounds are evaluated using a cell-based reporter gene assay.
Fig. 1.
Structures of compounds 1–5 isolated from Cornus alternifolia.
2. Materials and methods
2.1. Materials
Optical rotations were measured on a JASCO DIP-370 digital polarimeter equipped with a halogen lamp (589 nm) and a 10 mm microcell. IR spectra were recorded on a Nicolet Magna-IR 750 spectrophtotometer. NMR spectra were acquired with a Bruker Avance 400 spectrometer using solvent signals (Methanol-d4; δH 4.78/δC 49.15) as references. The ESI-HRMS data were obtained using an Agilent 1100 series TOF MS with electrospray ionization. Preparative HPLC was carried out using Agilent 1100 Series with Shim-park RP-C18 column (20 × 200 mm) and 1100 Series Multiple Wavelength detector.
The leaves of Cornus alternifolia were collected in Oxford, Mississippi, in September 2008 and voucher specimens (UM-2008008) were deposited at the Pullen Herbarium.
2.2. Extraction and isolation
The dried leaves of C. alternifolia (6.0 kg) were extracted with 90% methanol and dried in vacuo to to provide a crude extract. The crude extract (200 g) was sequentially separated on silica gel eluted by a step gradient of hexanes-ethyl acetate (100:0, 80:20, 50:50 and 0:100) and ethyl acetate-methanol mixtures (80:20, 50:50 and 0:100) to afford seven fractions (A-G). Fractions E and F exhibited moderate PPARα/γ and LXR agonistic activities. Fr. E (16 g) was chromatographed on reversed-phase C18 silica gel eluting successively with a gradient of H2O/methanol (1:0 to 0:1) to give five sub-fractions (Fr. E1-E5). E2 was further chromatographed on a silica gel eluted with chloroform/methanol in gradient (1:0 to 1:1) to obtain five sub-fractions (Fr. E2a-E2e), then Fr. E2c was separated by reversed-phase HPLC [Shim-park RP-C18 column; 5 μ m; 20 × 250 mm; step gradient from 35% methanol in H2O (0.02% HCOOH) to 50% methanol in H2O (0.02% HCOOH) for 90 min, 9 mL/min] to give 2 (4 mg, tR 49.6 min ), 3 (2 mg, tR 61.7 min) and 4 (5 mg, tR 30.5 min). Fr. F (65 g) was chromatographed over a silica gel column by eluting with chloroform/methanol (5:1 to 1:2) to give eight sub-fractions (Fr. F1-F8). Fr. F6 was separated on preparative RP HPLC (from 20% methanol in H2O to 35% methanol in H2O for 40 min, and followed by 35–65% methanol in H2O for 20 min, 12 mL/min) to afford nine sub-fractions (Fr. F6a-F6I), then Fr. F6g was purified by RP HPLC (Polor-C8; 5 μ m; 10 × 250 mm; 20–35% methanol in H2O for 90 min, 3 mL/min) to give 1 (10 mg).
Alternoside A (1)
white amorphous powder; mp 96–98 °C; ; IR (KBr) νmax: 3389, 2914, 1720, 1160, 1072, 1039 cm−1; NMR data (400 MHz, DMSO-d6, 25 °C, TMS,): see Table 1; ESI-HRMS m/z 413.1432 [M+Na]+ (calcd for C17H26O10Na, 413.1418).
Table 1.
NMR Data of 1 (DMSO-d6) and 2 (MeOD-d4), Measured at 400 MHz (1H) and 100 MHz (13C), δ in ppm, J in Hz..
| no. | 1
|
2
|
||||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | HMBC | NOESY | δH | δC | HMBC | NOESY | |
| 1 | 171.0 | H9, H3β | 5.31 d (4.8) | 96..5 | H1′, H3, H9 | H8 | ||
| 3a | 4.28 dd (10.4, 5.5) | 69.5 | H4, H5, H3α | 7.28 s | 153.1 | H1, H5 | ||
| 3b | 4.07 dd (10.4, 8.4) | H3β | ||||||
| 4 | 1.95 m | 43.3 | H3α, H3β | H5, H3β | 106.3 | H3, H5 | ||
| 5 | 2.89 ddd (5.1, 10.8, 15.6) | 42.9 | H9 | H4, H6, H9 | 3.29 d (7.2) | 43.8 | H1, H3 | H7β, H9, H10 |
| 6 | 3.99 m | 81.5 | H1′, H9 | H5, H7β, H9 | 215.9 | |||
| 7a | 2.16 dd (5.5, 12.5) | 42.7 | H10 | H6 | 2.42 dd (8.4, 18.7) | 44.6 | H10 | H8 |
| 7b | 1.36 ddd (3.1, 12.5, 14.9) | H6 | 1.89 dd (7.7, 18.7) | H8, H10 | ||||
| 8 | 1.86 m | 33.7 | H10 | H12, H7α | 2.23 m | 30.7 | H1, H5, H7α,β, H-10 | H1, H7α, H10 |
| 9 | 4.23 dd (3.9, 10.4) | 45.1 | H5 | H5 | 2.14 ddd (4.8, 7.0, 11.9) | 46.6 | H5, H7α,β, H8, H10 | H5, H7α |
| 10 | 1.02 d (6.4) | 18.6 | H7β, H8 | 1.14 d (6.7) | 20.0 | H7α,β, H8, H9 | H5, H7α,β, H8 | |
| 11 | 169.1 | H12 | 168.6 | H3, H12 | ||||
| 1′ | 3.97 d (7.5) | 104.6 | H2′ | H2′ | 4.78 d (8.5) | 98.6 | H1, H2′ | |
| 2′ | 2.95 dd (8.4, 16.2) | 73.7 | H1′, H3′ | 4.72 m | 74.4 | H3′ | ||
| 3′ | 3.09 dd (8.2, 16.9) | 76.2 | H2′ | H2′ | 3.48 dd (4.8, 17.8) | 76.0 | H2′ | |
| 4′ | 3.01 dd (9.4, 16.9) | 70.0 | H3′ | H6′ | 3.30 dd (1.5, 5.5) | 71.8 | H3′ | |
| 5′ | 3.03 dd (8.7, 16.9) | 76.9 | H4′ | H6′, H1′ | 3.30 dd (1.5, 5.5) | 78.7 | H1′, H6′α, H4′ | |
| 6a′ | 3.63 d (12.0) | 61.0 | 3.87 d (11.8) | 62.8 | H5′ | |||
| 6b′ | 3.41 dd (5.0, 11.6) | 3.61 dd (4.3, 10.4) | ||||||
| α | 5.65 d (12.8) | 116.4 | Hβ | |||||
| β | 6.79 d (12.8) | 145.8 | H2″, Hα | Hα | ||||
| 1″ | 7.61 d (8.7) | 127.7 | H3″, H5″, Hα | |||||
| 2″ | 6.68 d (8.7) | 134.2 | H6″, H β | H3″ | ||||
| 3″ | 115.9 | H2″, H5″ | H2″ | |||||
| 4″ | 160.3 | H2″, H3″, H6″ | ||||||
| 5″ | 6.68 d (8.7) | 115.9 | H3″, H6″ | H6″ | ||||
| 6″ | 7.61 d (8.7) | 134.2 | H3″, Hβ | H5″ | ||||
| O | 3.62 s | 3.48 s | 52.0 | |||||
| Me-CO | 167.0 | H2′, Hα,β | ||||||
Alternoside B (2)
colorless oil; ; IR (KBr) νmax: 3340, 1631, 1170, 1076, 760 cm−1; NMR data (400 MHz, MeOD-d4, 25 °C, TMS): see Table 1; ESI-HRMS m/z 557.1650 [M+Na]+ (calcd for C26H30O12Na, 557.1630).
Alternoside C (3)
colorless oil; ; IR (KBr) νmax: 3337, 1631, 1173, 1075, 764 cm−1; NMR data (400 MHz, MeOD-d4, 25 °C, TMS): see Table 2; ESI-HRMS m/z 557.1623 [M+Na]+ (calcd for C26H30O12Na, 557.1630).
Table 2.
NMR Data of 3 and 4. Both measured at 400 MHz (1H) and 100 MHz (13C) in MeOD-d4. δ in ppm, J in Hz..
| no. | 3
|
4
|
||||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | HMBC | NOESY | δ H | δ C | HMBC | NOESY | |
| 1 | 5.43 d (3.4) | 95.6 | H1′, H3 | H8 | 39.4 | H2α,β, H11, H12 | ||
| 2a | 2.37 d (20.9) | 56.7 | H11, H12 | H11 | ||||
| 2b | 1.98 d (2.3) | H11, H12 | ||||||
| 3 | 7.24 s | 152.7 | H1, H5 | 214.1 | H2α,β | |||
| 4a | 106.4 | H3, H5 | 2.27 m | 50.1 | H13 | H5, H13 | ||
| 4b | 2.10 m | H13 | ||||||
| 5 | 3.20 d (7.2) | 43.8 | H1, H3 | 1.92 m | 34.8 | H4α, H13 | H4β, H11 | |
| 6 | 214.8 | H5, H7α,β | 1.91 m | 58.1 | H5, H7 | H12, H13 | ||
| 7a | 2.42 dd (8.3, 18.5) | 45.0 | H10 | H8 | 5.45 dd (3.2, 6.4) | 134.1 | H6, H10, H11 | |
| 7b | 1.89 dd (8.1, 17.6) | H8, H10 | ||||||
| 8 | 2.16 m | 30.9 | H1, H5, H10 | H1, H7α, H10 | 5.44 dd (3.2, 6.4) | 136.8 | H10 | H1′, H9 |
| 9 | 2.12 m | 47.1 | H7β | 4.43 m | 75.1 | H1′, H8, H10 | H8, H10 | |
| 10 | 1.13 d (6.3) | 19.6 | H7α,β, H8, H9 | H5, H7α,β, H8 | 1.23 d (6.3) | 22.6 | H8, H11 | |
| 11 | 168.1 | H3, H12 | 0.91 s | 31.1 | H2α,β, H5, H7, H10 | |||
| 12 | 0.74 s | 21.6 | H6, H7, H13 | |||||
| 13 | 0.94 d (6.4) | 22.3 | H6 | |||||
| 1′ | 4.81 m | 98.4 | H1, H2′ | 4.32 d (7.7) | 101.0 | H2′ | ||
| 2′ | 4.75 m | 74.4 | H3′ | 3.14 m | 75.1 | H1′ | ||
| 3′ | 3.55 dd (9.5, 17.9) | 76.0 | H2′ | 3.23 m | 78.3 | H2′ | ||
| 4′ | 3.32 m | 71.8 | H3′ | 3.22 m | 71.8 | |||
| 5′ | 3.34 m | 78.8 | H4′ | 3.13 m | 78.4 | H1′ | ||
| 6a′ | 3.88 d (10.4) | 62.8 | H5′ | 3.80 dd (2.1, 11.9) | 63.0 | |||
| 6b′ | 3.63 dd (6.1, 11.8) | 3.58 dd (6.0, 11.9) | ||||||
| α | 6.21 d (17.0) | 115.0 | Hβ | Hβ | ||||
| β | 7.53 d (17.0) | 146.9 | H2″, H6″ | Hα | ||||
| 1″ | 127.4 | H3″, H5″, Hα | ||||||
| 2″ | 7.40 d (8.6) | 131.5 | H6″, Hβ | H3″ | ||||
| 3″ | 6.75 d (8.6) | 117.0 | H5″ | H2″ | ||||
| 4″ | 161.5 | H2″, H6″ | ||||||
| 5″ | 6.75 d (8.6) | 117.0 | H3″ | H6″ | ||||
| 6″ | 7.40 d (8.6) | 131.5 | H2″, Hβ | H5″ | ||||
| O | 3.19 s | 51.9 | ||||||
| Me-CO | 168.0 | H2′, Hα,β | ||||||
Alternoside D (4)
colorless amorphous powder; mp 113–115 °C; ; IR (KBr) νmax: 3337, 1631, 1173, 1075, 764 cm−1; NMR data (400 MHz, MeOD-d4, 25 °C, TMS): see Table 2; ESI-HRMS m/z 395.2078 [M+Na]+ (calcd for C19H32O7Na, 395.2077).
2.3. Acid Hydrolysis and Determination of the Sugar Configuration
Alternoside A (1) (2 mg) was hydrolyzed by heating in 1 M HCl (0.4 mL) at 100 °C for 2 h under an Ar atmosphere and neutralized with amberlite IR 400. After drying in vacuo, the residue was dissolved in pyridine (0.4 mL) containing L-cysteine ethyl ester hydrochloride (2 mg) and heated at 60 °C for 1 h. A 0.4 ml solution of 3, 5-dichlorophenyl isothiocyanate (2 mg) in pyridine was added to the mixture, which was heated at 60 °C for 1 h. The reaction mixture was directly analyzed by analytical HPLC (Shim-park RP-C18 column; 5 μ m; 4.6 × 250 mm) column by eluting with a gradient of 30–80% acetonitrile in H2O + 0.02% HCOOH for 40 min and subsequent washing of the column with 100% acetonitrile at a flow rate 0.8 mL/min. In the acid hydrolysate of 1, D-glucose was confirmed by comparison of the retention times of their derivatives with those of D-glucose and L-glucose derivatives prepared in the same way which showed retention times of 34.8 and 34.0 min, respectively. Sugars in 2–4 were also identified by the same method.
2.4 Determination of PPARα/γ and LXR agonistic activities
Cell-based luciferase reporter gene assays were used to evaluate PPARα/γ and LXR agonistic activities of compounds as described previously [13–15]. Human hepatoma (HepG2) cells and Chinese hamster ovary cells (CHO) cells were cultured in DMEM/Ham’s F12 medium supplemented with FBS (10%) and antibiotics (penicillin G sodium 100 U/mL and streptomycin 100 μg/mL) at 37 °C in an atmosphere of 95% humidity and 5% CO2. At about 75% confluence, cells were harvested by trypsinization and transfected with firefly luciferase reporter gene constructs containing PPARα and γ, and peroxisome proliferator response element (PPARE) in HepG2 cells and CHO cells, respectively. Briefly, 25 μg of DNA plasmids was added to 500 μL cell suspension (5 × 106 cells) and incubated for 5 min at room temperature in BTX disposable cuvettes (4 mm gap). The cells were electroporated at 150 V (HepG2 cells) or 155 V (CHO cells) and a single 70 ms pulse in a BTX Electro Square Porator T 820 (BTX I, San Diego, CA). Transfected cells were plated in 96-well plate at a density of 5 × 104 cells/well and grown for 24 h. The cells were treated with different concentrations of test compounds for 24 h followed by addition of 40 μ L 1:1 mixture of Luc-Lite reagent and PBS containing 1 mmol calcium and magnesium. Luciferase activity was determined in terms of light output measured on a TopCount microplate reader (Packard Instrument Co. Meriden, CT) in a single photon counting mode. The pSV-β-galactosidase control plasmid (Promega, USA) was used to normalize the transfection efficiencies.
For LXR agonistic activity, 5 × 106 of CHO cells were harvested and transiently transfected with the reporter gene constructs LXR and LXRE using electroporation (155 V, 70 mV, 1 pulse). After transfection, cells were transferred into 96 well microtiter plates and cultured for 24 h. Cells were washed once with Ham’s F12 basic medium and were cultured in Ham’s F12 medium with 5% newborn calf lipoprotein deficient serum (NCLDS) and 20 μg/mL of LDL. Drug treatment and luciferase assays were identical to PPAR assay. EC50 values were calculated using Microsoft Excel software. The ciprofibrate, ciglitazone and 25-hydroxyl-cholesterol were used as positive control for PPARα, PPARγ and LXR respectively.
3. Results and discussion
A 90% methanol extract of the dried leaves of C. alternifolia (6 kg) was sequentially separated on silica gel eluted by a gradient of hexanes-ethyl acetate (100:0, 80:20, 50:50 and 0:100) and ethyl acetate-methanol mixtures (80:20, 50:50 and 0:100) to afford seven fractions (A-G). Bioassay-guided fractionation of fractions D and E were further chromatographed over a silica gel and then a series of HPLC separations on C-18 and C-8 silica gel to yield compounds (1–4).
Alternoside A (1) was isolated as a white amorphous powder. Its molecular formula was deduced to be C17H26O10, with five degrees of unsaturation, from an ion m/z 413.1432 (ESI-HRMS). The IR spectrum exhibited broad absorption for multiple OH groups (3388 cm−1) and a lactone carbonyl (1720 cm−1) functionality. Analysis of the 1H and 13C NMR spectroscopic data (see Table 1) indicated signals for one glucose moiety, a carbomethoxy group, an oxymethine, four other methines, an oxymethylene, a methylene and a methyl. The signals at δC 104.6, 76.9, 76.2, 73.7, 70.0 and 61.0 were characteristic for a β-glucopyranosyl moiety [16].
These data and a lactone carbonyl (δC 171.0) led to the preliminary conclusion that the molecular skeleton of compound 1 was similar to jataminin G [17]. Analysis of the 1H-1H COSY spectrum of 1 showed the presence of two spin systems involving the protons at C-9, C-5, C-6, C-7, C-10 and C-5, C-4, and C-3. Further analysis of HMQC and HMBC NMR data (see Figure S29 in the supporting information) allowed assignment of all proton and carbon signals. Key HMBC correlations from Me-8 to C-8, C-7, and C-9, and from both H-5 and H-3a to C-1 established the planar structure of 1. Furthermore, HMBC correlation from H-1′ to C-6 confirmed the glucosylation at C-6.
The relative configuration of compound 1 was determined by comparison of chemical shifts and coupling constants to the literature data and by analysis of NOESY correlations (Figure S30 in the supporting information). The relationship of H-5 to H-9 was determined to be cis from the coupling constant (10.4 Hz), which is larger in trans-fused iridoids (~ 12–13 Hz). Assuming the usual iridoid glucoside, the configuration of H-5 and H-9 is β [18]. The quasi-equatorial positions of both H-4 and H-6 were confirmed by the small coupling constants of JH4,H3ax 5.5 Hz, and JH4,H3ex 8.4 Hz, JH7,H7ax 5.5 Hz and JH6,H7ex 12.5 Hz [17], respectively. This assignment was also supported by NOESY correlations from the H-5 to H-4 and H-6, H-6 to Hβ-7 and H-9, and Hβ-7 to Me-10. Thus, the structure of 1 was characterized as (4S,5R,6S,8S,9R)-nepetalactone-6-O-β-D-glucopyranoside.
6-Glucosyl-substituted iridolactones are rare. A few iridolactones have been reported from species of the genera Valeriana [17] and Patrinia [19]. However, they typically lack a glucosyl moiety or have a glucosyl moiety at position 7 or 8. Thus, 1 is the first reported iridoid glucoside with a 6-O-β-D-glucopyranosyl moiety.
Alternoside B (2) was isolated as colorless oil with the molecular formula C26H30O12, as determined by ESI-HRMS. The 1H and 13C NMR data (Table 1) of 2 were similar to those of cornin [20] except for the presence of new signals in the aromatic region consistent with a p-substituted phenylpropanoid. In the HMBC spectrum of 2, the correlation between the methyl proton at δH 3.48 (MeO-11) and the carbonyl carbon at δC 168.6 (C-11) confirmed that the methyl group was part of a methyl ester at C-4 (δC 106.4). Thus, the phenylpropanoid moiety was shown to be a p-coumaryl ester and not a p-methyoxycinnamoyl ester. The coupling constant (12.8 Hz) between H-α and H-β indicated that it was the Z isomer of the p-coumaroyl ester. The ester was located at C-2′ of the glucosyl moiety on the basis of the downfield signal of H-2′ (δH 4.72 ppm) in the 1H NMR spectrum and a clear 3J HMBC correlations of H-2′ to carbonyl carbon of the p-coumaryl. The glycosyl moiety at C-1 (δC 95.6) was confirmed by the HMBC correlation from H-1′ to C-1. The relative configuration of 2 was confirmed as described for 1. Furthermore, correlations from H-1 to H-8 and H-8 to Hα-7 revealed that those protons adopted α orientation. Thus, alternoside B (2) was determined to be 2′-O-Z-p-coumaryl-cornin.
Alternoside C (3) was shown to have the same molecular formula as 2, C26H30O12, by the ESI-HRMS. The 1H and 13C NMR data (see Table 2) of 3 coincided well with those of 2 except for the coupling constant (17.0 Hz) of H-α and H-β of p-coumaryl, indicated the E isomer of the p-coumaryl group. Hence, compound 3 was identified as 2′-O-E-p-coumaryl-cornin.
Cornoside E (4) was obtained as colorless amorphous powder. Its molecular formula of C19H32O7 was determined by the ESI-HRMS. The IR absorption band at 3327.0 and 1637.7 cm−1 suggested the presence of hydroxyl and ketone group. The 1H and 13C NMR spectra (see Table 2) of 4 showed signals for a β-D-glucopyranosyl unit. The chemical shifts of the remaining 13 peaks in 13C NMR spectrum indicated a megastigmane aglucone, and the presence of a carbonyl signal at δC 214.1 suggested a structure similar to that of (5S,6R)-9-hydroxymegastigm-7-en-3-one [21] except for the coupling constant between H-7 and H-8 changed from 15.4 Hz to 5.1 Hz for compound 4, which indicated the aglycone of 4 to be a 9-hydroxymegastigm-7-Z-en-3-one. The relative configuration of 4 was assigned using the NOESY experiment. Correlations from H-5 to CH3-11 suggested that both H-5 and CH3-11 had axial orientation, while the cross-peaks between H-7 and CH3-11, H-5 indicated the side chain must be in the equatorial orientation [22]. Configuration at C-9 was assigned to be S on the basis of the 13C NMR signal at δC 75.1 (δC 77.0 for C-9 in R-form and δC 74.7 for C-9 in S-form) [23]. The absolute configuration of C-5, 6 was not determined. This finding suggested that 4 was (5*R,6S*,9S,7Z)-megastigmane-3-one-7-en-9-O-β-D-glucopyranoside.
The monosaccharides for compounds 1–4, were obtained after acid hydrolysis of each compound and identified as glucose by LC-MS comparison with an authentic sample. The D absolute configuration of glucose was confirmed by LC-MS analysis of chiral derivatives from the hydrolysate (see Experimental Section). The relatively large coupling constants (6.0–8.0 Hz) for the anomeric protons in the 1H NMR spectra (in Tables 1 and 2) of these compounds suggested that the glucopyranosyl moieties have a β-configuration.
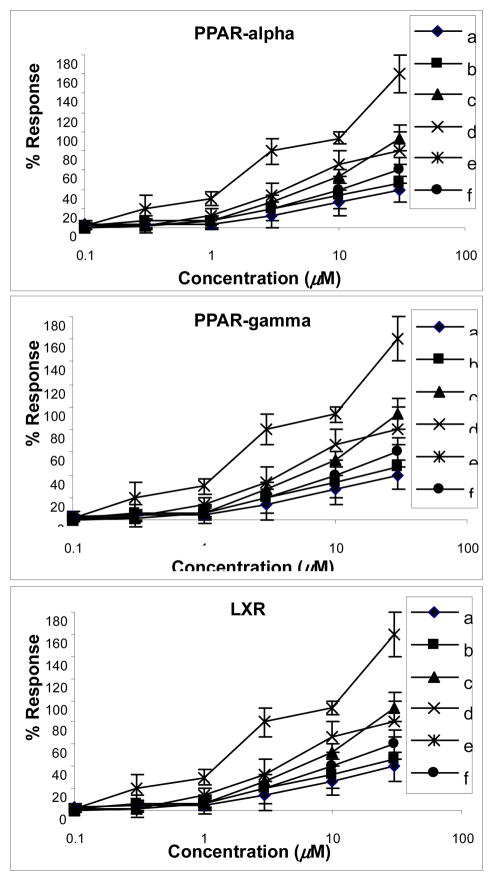
The new compound 1 and the known compounds 8-epihastatoside [16], olean-12-en-28-oic acid [24], arjungenin [25], kaempferol-3-O-β-D-glucopyranoside [26], and ellagic acid-4-O-β-D-xylopyranoside-3,3′-dimethyl ether [27] were evaluated for agonistic activity for PPAR-α/γ and LXR (Fig. 2). The results indicated that kaempferol-3-O-β-D-glucopyranoside showed significant activity for PPARα, PPARγ and LXR with the EC50 values of 0.62, 3.0 and 1.8 μM respectively. Compounds olean-12-en-28-oic acid, and ellagic acid-4-O-β-D-xylopyranoside-3,3′-dimethyl ether showed moderate agonistic activity for PPARα and LXR. Arjungenin showed moderate selective activity for PPARα; however, compound 1 and 8-epihastatoside showed only weak activity on LXR and no activity for PPAR-α/γ. The other compounds revealed no activity for PPAR-α/γ nor LXR at the maximum concentration of 30 μM. A luciferase construct with binding sites for Sp-1 was used as a control. The above compounds showed no activation or inhibiting activity on Sp-1 (data not shown). These results suggest that kaempferol-3-O-β-D-glucopyranoside has potential for the treatment of diabetes and further investigations are warranted.
Fig. 2.
Effects of iridoid and megastigmane glycosides from Cornus alternifolia on PPARα, PPARγ and LXR. Results were expressed as percent response of ciprofibrate (30 μ M), ciglitazone (10 μ M) and 25-hydroxyl-cholesterol (10 μ M), respectively, with respect to luciferase activity assessed by light production. Data were obtained from 3 experiments in duplicate. a: alternoside A, b: 8-epihastatoside, c: olean-12-en-oic acid, d: arjungenin, e: kaempferol-3-O-β-D-glucopyranoside, f: 4-O-β-D-xylopyranoside-3,3′-dimethyl ether.
Kaempferol-3-O-β-D-glucopyranoside is an important chemotaxonomic marker and occurs widely in food plants such as blank beans (Phaseolus vulgaris L.; yield of 0.185%), which are widely consumed throughout the world [28]. Jussiaea repens L. [29] and other edible medicinal herbs, such as Eucommia ulmoides [30], Rubus ulmifolius [31], Pistasia integerrioma [32], and Rosa canina [33] provide much lower yields of 0.00193%, 0.00045%, 0.00044%, 0.00193% and 0.00089%, respectively. Kaempferol-3-O-β-D-glucopyranoside has also has been found to have a mild inhibiting effect on the proliferation of HepG2 cells with an EC50 value of 306.4 ± 131.3 μM [28] and significant glycation inhibitory activity of bovine serum albumin (BSA) with an IC50 value of 0.32 μM [30], DPPH radical scavenging activity (IC50 = 86.10 μ M) and xanthine oxidase inhibitory activity (IC50 = 21.20 μ M) [32] potential effects to reduce the gain of body weight and visceral fat [33] the ONOO− scavenging activity (inhibitory activity of authentic peroxynitrite) with IC50 values of 6.98 ± 0.37 μ M [34]. Furthermore, kaempferol-3-O-β-D-glucopyranoside moiety has been found to be essential for the bioactivity and effect of the flavonol glycosides on the inhibition of tumor necrosis factor-α (TNF-α) production, decreased sensitivity of hepatocytes to TNF-α, and on the protection of hepatocytes against D-galactosamine (D-GalN) [35].
Supplementary Material
Highlights.
The metabolites isolated show potential active agonists for PPAR and LXR receptors.
Four new natural products 1–4 were isolated from the leaves of Cornus alternifolia.
Metabolite 1 is the first iridoid glycoside containing a β-glucopyranoside at C-6.
Metabolite 5 showed potent agonistic activities for PPARα, PPARγ and LXR.
The results presented can be used for further synthetic and pharmacological studies.
Acknowledgments
Financial support for this project was provided by NIAID 5RO1AI1036596, an NIH research facilities improvement grant C06 RR-14503-01 and young teacher training of the Xi’an University of Technology awarded to Yang-Qing He, and by the Scientific Research Program Funded by Shaanxi Provincial Education Department (2010JK749). We are also grateful to Bin Wang for her valuable discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Beltowski J. Liver X receptors (LXR) as therapeutic targets in dyslipidemia. Cardiovasc Ther. 2008;26:297–316. doi: 10.1111/j.1755-5922.2008.00062.x. [DOI] [PubMed] [Google Scholar]
- 3.Makishima M. Nuclear Receptors as Targets for Drug Development: Regulation of Cholesterol and Bile Acid Metabolism by Nuclear Receptors. J Pharmacol Sci. 2005;97:177–183. doi: 10.1254/jphs.fmj04008x4. [DOI] [PubMed] [Google Scholar]
- 4.Staels B, Fruchart JC. Therapeutic Roles of Peroxisome Proliferator–Activated Receptor Agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 5.Berger J, Moller DE. The Mechanisms of Action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 6.Vanwijk JP, Dekoning EJ, Marterns EP, Rabelink TJ. Thiazolidinediones and Blood Lipids in Type 2 Diabetes. Arterioscler Thromb Vasc Biol. 2003;23:1744–1749. doi: 10.1161/01.ATV.0000090521.25968.4D. [DOI] [PubMed] [Google Scholar]
- 7.Fan C, Xiang QY. Phylogenetic relationships within Cornus (Cornaceae) based on 26S rDNA sequences. Am J Bot. 2001;88:1131–1138. [PubMed] [Google Scholar]
- 8.Vareed SK, Reddy MK, Schutzki RE, Nair MG. Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits. Life Sci. 2006;78:777–784. doi: 10.1016/j.lfs.2005.05.094. [DOI] [PubMed] [Google Scholar]
- 9.Seeram NP, Schutzki R, Chandra A, Nair MG. Characterization, Quantification, and Bioactivities of Anthocyanins in Cornus Species. J Agric Food Chem. 2002;50:2519–2523. doi: 10.1021/jf0115903. [DOI] [PubMed] [Google Scholar]
- 10.Wang HB, Nair MG, Strashurg GM, Chang YC, Booren AM, Gray JI, Dewitt DL. Antioxidant and Antiinflammatory Activities of Anthocyanins and Their Aglycon, Cyanidin, from Tart Cherries. J Nat Prod. 1999;62:294–296. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- 11.Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, Raskin I, Lila MA. Hypoglycemic activity of a novel anthocyanin-rich formulation from Lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16:406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen P, Liu MH, Ng TY, Chan YH, Yong EL. Differential effects of isoflavones, from Austragalus membranaceus and Pueraria Thomsonii, on the activation of PPARα, PPARγ, and adipocyte differentiation in vitro. J Nutr. 2006;136:899–905. doi: 10.1093/jn/136.4.899. [DOI] [PubMed] [Google Scholar]
- 14.Pavlov TS, Levchenko V, Karpushev AV, Vandewalle A, Staruschenko A. Peroxisome proliferator-activated receptor γ antagonists decrease Na+ transport via the epithelial Na+ channel. Mol Pharmacol. 2009;76:1333–1340. doi: 10.1124/mol.109.056911. [DOI] [PubMed] [Google Scholar]
- 15.Huang TH, Razmovski-Naumovski V, Salam NK, Duke RK, Tran VH, Duke CC, Roufogalis BD. A novel LXR-α activator identified from the natural product Gynostemma pentaphyllum. Biochem Pharmacol. 2005;70:1298–1308. doi: 10.1016/j.bcp.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Franzyk H, Jensen SR, Olsen CE, Quiroga JM. A 9-Hydroxyiridoid Isolated from Junellia seriphioides (Verbenaceae) Org Lett. 2000;2:699–700. doi: 10.1021/ol0055521. [DOI] [PubMed] [Google Scholar]
- 17.Lin S, Chen T, Liu XH, Shen YH, Li HL, Shan L, Liu RH, Xu XK, Zhang WD, Wang H. Iridoids and Lignans from Valeriana jatamansi. J Nat Prod. 2010;73:632–638. doi: 10.1021/np900795c. [DOI] [PubMed] [Google Scholar]
- 18.Ayers S, Sneden A. Caudatosides A-F: New Iridoid Glucosides from Citharexylum caudatum. J Nat Prod. 2002;65:1621–1626. doi: 10.1021/np020211c. [DOI] [PubMed] [Google Scholar]
- 19.Kouno I, Yasuda I, Mizoshiri H, Tanaka T, Marubayashi N, Yang DM. Two new iridolactones and their glycosides from the roots of Patrinia scabra. Phytochemistry. 1994;37:467–472. doi: 10.1016/0031-9422(94)85081-x. [DOI] [PubMed] [Google Scholar]
- 20.Franzyk H, Jensen SR, Stermita FR. Iridoid glucosides from Penstemon secundiflorus and grandiflorus: Revised structure of 10-hydroxy-8-epihastatoside. Phytochemistry. 1998;49:2025–2030. [Google Scholar]
- 21.Sefton MA, Francis L, Williams PJ. Volatile norisoprenoid compounds as constituents of oak woods used in wine and spirit maturation. J Agric Food Chem. 1990;38:2045–2049. [Google Scholar]
- 22.Otsaka H, Zhong XN, Hirata E, Shinzato T, Tokenda Y. Myrsinionosides A-E: Megastigmane Glycosides from the Leaves of Myrsine seguinii LEV. Chem Pharm Bull. 2001;49:1093–1097. doi: 10.1248/cpb.49.1093. [DOI] [PubMed] [Google Scholar]
- 23.Marino SD, Borbone N, Zollo F, Ionaro A, Meglio PD, Iorizzi M. Megastigmane and Phenolic Components from Laurus nobilis L. Leaves and their Inhibitory Effects on Nitric Oxide Production. J Agric Food Chem. 2004;52:7525–7531. doi: 10.1021/jf048782t. [DOI] [PubMed] [Google Scholar]
- 24.Seebacher W, Simic N, Weis R, Saf R, Kunert O. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn Reson Chem. 2003;41:636–638. [Google Scholar]
- 25.Jossang A, Seuleiman M, Maidou E, Bodo B. Pentacyclic triterpenes from Combretum nigricans. Phytochemistry. 1996;41:591–594. [Google Scholar]
- 26.Matsuura S, Linuma M. Studies on the Constituents of useful Plants. VI. Constituents of the Calyx of Diospyros kaki (2), and Carbon-13 Nuclear Magnetic Resonance Spectra of Flavonol Glycosides. Chem Pharm Bull. 1978;26:1936–1941. [Google Scholar]
- 27.Castaneda P, Bahena A, Garcia E, Chavez D, Mata R. Secondary Metabolites from the Stem Bark of Celaenodendron mexicanum. J Nat Prod. 1993;56:1575–1579. [Google Scholar]
- 28.Dong M, He XJ, Liu RH. Phytochemicals of Black Bean Seed Coats: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidative Activities. J Agric Food Chem. 2007;55:6044–6051. doi: 10.1021/jf070706d. [DOI] [PubMed] [Google Scholar]
- 29.Huan HL, Li DL, Li XM, Xu B, Wang BG. Antioxidative principals of Jussiaea repens: an edible medicinal plant. Int J Food Sci Tech. 2007;42:1219–1227. [Google Scholar]
- 30.Kim HY, Moon BH, Lee HJ, Choi DH. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. J Ethnopharmacol. 2004;93:227–230. doi: 10.1016/j.jep.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 31.Acqua SD, Cervellati R, Loi MC, Innocenti G. Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: Investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 2008;106:745–749. [Google Scholar]
- 32.Ahmad NS, Farman M, Najmi MH, Mian KB, Hasan A. Pharmacological basis for use of Pistacia integerrima leaves in hyperuricemia and gout. J Ethnopharmacol. 2008;117:478–482. doi: 10.1016/j.jep.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya K, Matsuda H, Kubo M, Morikawa T, Nishida N, Yoshikawa M. Potent anti-obese principle from Rosa canina: Structural requirements and mode of action oftrans-tiliroside. Bioorg Medicinal Chem Lett. 2007;17:3059–3064. doi: 10.1016/j.bmcl.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 34.Jung HA, Kim JE, Chung HY, Choi JS. Antioxidant principles of Nelumbo nucifera stamens. Arch Pharm Res. 2003;26:279–285. doi: 10.1007/BF02976956. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda H, Ninomiya K, Shimoda H, Yoshikawa M. Hepatoprotective principles from the flowers of Tilia argentea (Linden): structure requirements of tiliroside and mechanisms of action. Bioorg Medicinal Chem. 2002;10:707–712. doi: 10.1016/s0968-0896(01)00321-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.