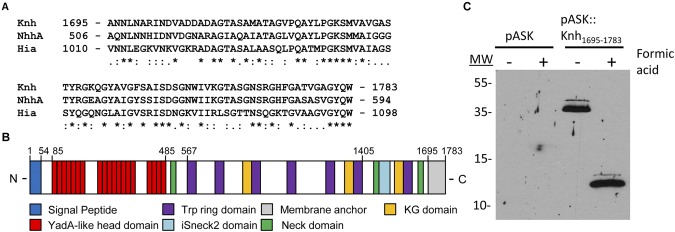
FIG 1 .
K. kingae Knh is a trimeric autotransporter. (A) Knh C-terminal membrane anchor amino acid sequence alignment with N. meningitidis NhhA and H. influenzae Hia. Asterisks indicate identical residues, colons indicate conserved substitutions, and periods indicate semi-conserved substitutions. (B) Annotation of Knh, highlighting the presence of numerous protein domains, including a signal peptide, a membrane anchor, YadA-like head domains, Trp ring domains, and an ISneck2 domain. (C) Strep-tag Western blot analysis of outer membrane preparations and formic acid-denatured outer membrane preparations following the induction of vector control pASK-IBA12 or pASK::Knh1695-1783, which expresses the Knh beta-barrel domain fused to a Strep-tag. Molecular sizes (MW) are expressed in kilodaltons.