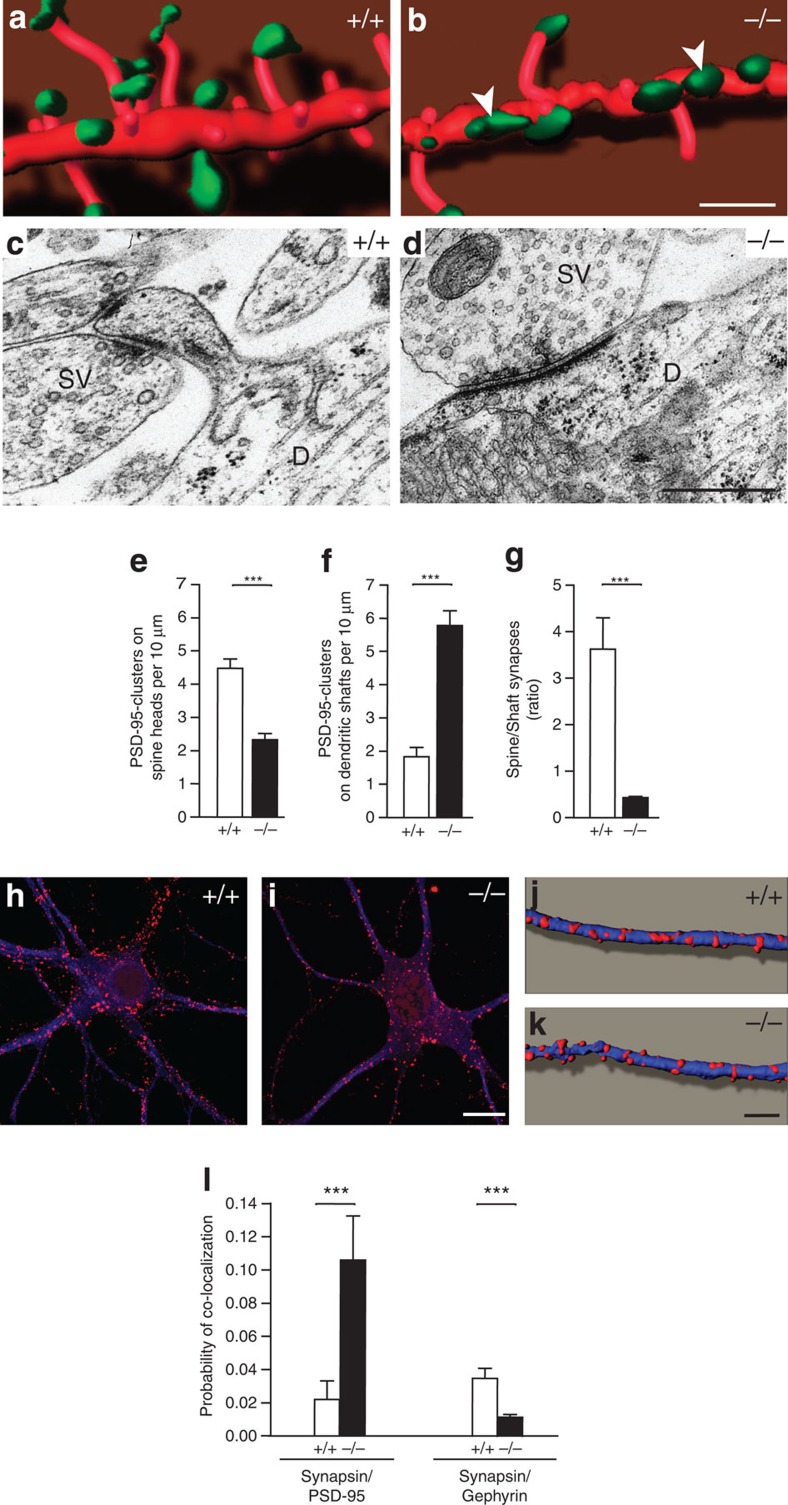
Figure 3. Organization of the postsynaptic compartment in absence of Nbea.
(a,b) Spine-bearing dendrites from mRFP-transfected wild-type (+/+) and knockout (−/−) neurons in vitro (red) were co-stained against PSD-95 (green), and stacks of confocal images were three-dimensionally reconstructed using Imaris software. Arrowheads point to PSD-95 clusters that form directly at dendritic shafts of mutant neurons. (c,d) Sample electron micrographs of axo-spinous (c) and axo-dendritic synapses (d) as observed in control and mutant hippocampal neurons. SV, synaptic vesicles; D, dendrite. (e–g) Quantitative analysis of PSD-95 clusters, averaged from 25–29 dendrites from 3–4 independent cultures per genotype, localized on spine heads (e) versus those on dendritic shafts (f), and their ratio in neuronal cultures of both genotypes at DIV21 (g). (h,i) Distribution of the inhibitory postsynaptic scaffolding molecule gephyrin (red) on somata and dendrites of neurons from both genotypes (co-labelling against MAP2, blue). (j,k) Three-dimensional reconstructions from stacks of confocal images as those shown in panel h,i. (l) Degree of co-localization between synapsin-positive punctae with PSD-95 clusters was increased in KO neurons as seen by an increased probability of co-localization (synapsin/PSD-95). In contrast, the probability of co-localization of synapsin with gephyrin was reduced (synapsin/gephyrin), as quantitated on 44–47 dendrites from four independent cultures per genotype. All data are means±s.e.m. ***P<0.001. Scale bars, 5 μm in a, b, 500 nm in c, d, 10 μm in h–i and 5 μm in j–k.