Abstract
We present a rare case of a gastrointestinal stromal tumor (GIST) in the stomach with prominent calcification at presentation. A 61-year-old woman visited our hospital because of epigastric discomfort. A spherical calcified lesion with a diameter of about 30 mm was incidentally shown in the left upper quadrant on an abdominal X-ray. Computed tomography demonstrated that the tumor was growing from the upper gastric body, with calcification in the peripheral ring area. A laparoscopic partial gastrectomy was performed, and the resected specimen revealed a well-circumscribed tumor with exophytic growth from the gastric muscularis propria. Microscopic examination revealed spindle-shaped tumor cells with calcification and hemorrhage. Additionally, positive immunoreactivity of the tumor to KIT and CD34 and a low mitotic index resulted in the diagnosis of very low risk GIST. There are a few case reports of heavily calcified GIST, although solitary or punctate calcification of primary GIST has been reported in several case series. Dystrophic calcification of necrotic or degenerative tissue is the supposed cause of primary calcified GISTs. In contrast, appearance of calcification after administration of imatinib mesylate, which may be one indicator of disease response, is possibly caused by a different mechanism.
Keywords: Gastrointestinal stromal tumor, Calcification, Stomach, Computed tomography, Imatinib mesylate
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common primary mesenchymal neoplasms of the gastrointestinal tract[1,2]. In the past decade, histological and clinical features of GISTs have become increasingly clear[1,2], however, calcified tumor mass is an unusual feature of GISTs at initial presentation, and the mechanism is not clear. We describe here an uncommon case of gastric GIST with prominent calcification.
CASE REPORT
A 61-year-old woman without a significant medical history visited our hospital due to epigastric discomfort. There were no positive findings on physical examination. Initial laboratory tests demonstrated low hemoglobin level (9.6 g/dL), which was probably due to iron deficiency anemia.
An abdominal X-ray showed a spherical calcified lesion measuring approximately 30 mm in the left upper quadrant (Figure 1A). An upper gastrointestinal barium study revealed an exogastric mass (Figure 1B). A plain computed tomography (CT) demonstrated calcification in the peripheral ring area of the tumor growing from the upper gastric body. With an abdominal enhanced CT, the tumor was enhanced heterogeneously, except for the calcified section (Figure 2A and B). There was no evidence of liver metastasis or diffuse peritoneal spread. Magnetic resonance imaging of the central portion of the tumor showed low signal intensity on T1-weighted images (Figure 2C) and high signal intensity on T2-weighted images (Figure 2D). Indicative of calcification, peripheral portions showed low intensity on both T1 and T2-weighted images. Endoscopic examination revealed a round submucosal tumor with the upper part depressed (Figure 3A). Endoscopic ultrasonography (EUS) indicated the tumor originated from the fourth layer of the gastric wall (Figure 3B). Although EUS-guided fine needle aspiration was considered for the diagnosis of the submucosal lesion, it was impossible to perform due to calcification of the tumor wall with a sonic shadow (Figure 3B). After informed consent was obtained, laparoscopic partial gastric resection was performed.
Figure 1.
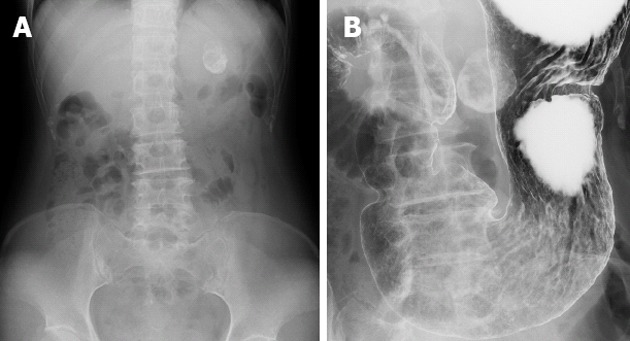
Radiological findings. A: Abdominal X-ray indicated a spherical calcified lesion in the left upper quadrant; B: Barium study showed a round calcification bordering the gastric wall.
Figure 2.
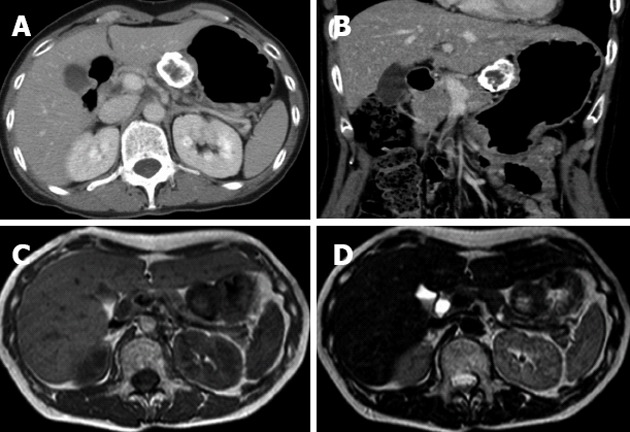
Computed tomography and magnetic resonance imaging findings. Contrast-enhanced axial (A) and coronal (B) computed tomography examination demonstrated that the marginal zone of the tumor was calcified, and that the internal portion of the tumor was enhanced heterogeneously. Magnetic resonance imaging T1-weighted image (C) and T2-weighted image (D) revealed a low intensity marginal zone of tumor reflecting calcification.
Figure 3.
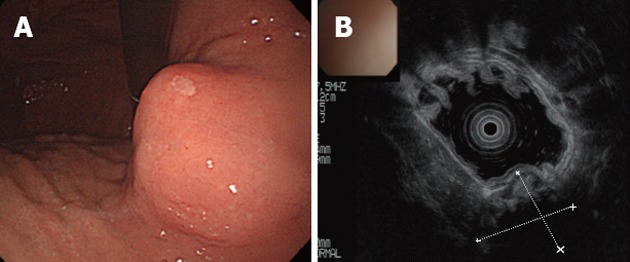
Endoscopy and endoscopic ultrasound of stomach. A: Endoscopic examination revealed a round submucosal tumor; B: The tumor originated from the fourth layer of the gastric wall as indicated by endoscopic ultrasound. The deeper section could not be visualized because of calcification.
The resected specimen revealed a well-circumscribed tumor measuring 32 mm × 23 mm × 23 mm originating from the gastric muscularis propria. The sliced surface of the tumor showed a firm, solid, whitish-gray parenchyma with circular calcification and internal bleeding (Figure 4A). Microscopic investigation revealed spindle-shaped tumor cells (Figure 4B) with calcification and hemorrhage. The cells exhibited low mitotic activity of fewer than 5 mitoses per 50 high power field (HPF) and no prominent signs of nuclear atypia. Immunohistochemical staining demonstrated positive reactivity to KIT (Figure 4C) and CD34 (Figure 4D). On the other hand, there was negative reactivity to S-100 protein, smooth muscle actin, and desmin (not shown). Since the mitotic index of this tumor was less than 5/50 HPF, its diagnosis was established as very low risk GIST according to risk stratification guidelines[1]. The patient had an uneventful postoperative course and was discharged on the 7th postoperative day. The patient has been followed up for 10 mo without any signs of recurrence.
Figure 4.
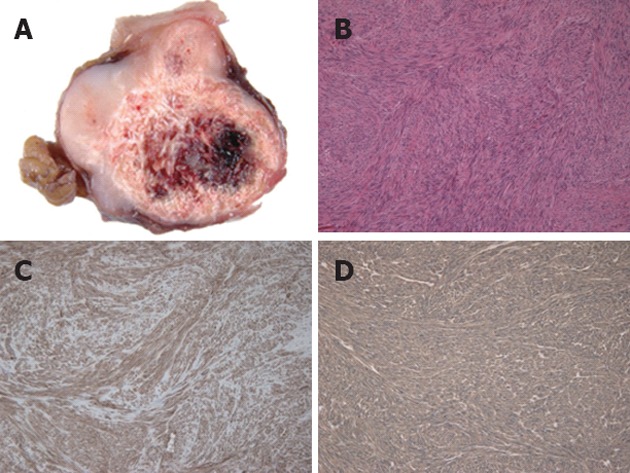
Pathological findings. A: Sliced sections of the resected mass demonstrated a firm, solid, whitish-gray parenchyma with circular calcification and internal bleeding; B: Microscopically, the tumor was characterized by spindle-shaped tumor cells (hematoxylin and eosin, original magnification ×100); Immunohistochemically, the tumor cells were positive for KIT (C) and CD34 (D).
DISCUSSION
GISTs are defined as mesenchymal tumors in the gastrointestinal tract that express KIT, a tyrosine kinase growth factor receptor[1]. Clinical, histological and molecular features of GISTs have become increasingly clear in the past decade[1,2], and radiologic appearances of these tumors have also been well described[2-7]. Small GISTs (< 5 cm) are often seen as well-defined with a homogeneous appearance on contrast-enhanced scans, whereas larger lesions (> 10 cm) are more likely to have irregular and lobulated margins with heterogeneous enhancement[2-4].
Although several cases of primary GIST with calcification have been reported[3-6], solitary or punctate calcification was shown in most cases[4,5]. In contrast, there are only six case reports describing heavily calcified GISTs[8-13]; three cases located in the stomach, one in the colon, and two in the rectum. Although Ghanem et al[4] referred to a solitary calcification as an aggressive finding, the evidence was not clearly shown. Regarding primary lesion, Tateishi et al[5] suggested that the frequency of calcification was not a reliable finding for the distinction of low- and high-grade GISTs. Particularly, with respect to malignant GIST of the stomach, no CT feature (including calcification) other than size was found to have predictive value[6].
The mechanism of calcification at presentation is unclear. Yoshida et al[8] suggested that the calcification of GIST may be developed mostly from necrotic tissue. In the earlier literature, a true GIST would be included in the imaging studies of leiomyoma, leiomyoblastoma, or leiomyosarcoma arising in the gastrointestinal tract. Prior to the appearance of the definition of GIST, the major mechanism of calcified gastric submucosal tumors was also supposed to be dystrophic calcification of necrotic or degenerative tissue[14].
In addition, several cases have demonstrated increasing calcification in mesenteric metastasis after therapy with imatinib mesylate[7,15]. Histological examination of liver metastasis after therapy showed myxomatous degeneration, leaving small pyknotic nuclei in an eosinophilic myxoid background, with no signs of an inflammatory reaction or necrosis[16,17]. Imatinib mesylate alters the balance from cellular proliferation to apoptosis by inhibiting the tyrosine kinase activity of KIT[16]. Because cellular death of GISTs following imatinib therapy is by apoptosis and not by necrosis, which is the principal mechanism of cell death following conventional cytotoxic chemotherapy[7], the mechanism of calcification following administration of imatinib is probably different from that of primary calcification.
[18F]-fluorodeoxyglucose-positron emission tomography has been reported as useful for the evaluation of early response to imatinib mesylate treatment[16,18,19]. Furthermore, a decrease in tumor size or a tumor density on post-treatment CT has been reported as a good predictor for tumor response[19,20]. Sandrasegaran et al[7] also reported that four of twenty-two mesenteric masses showed calcification as well as cystic changes following at least 6 mo of therapy. It is suggested that calcification, in addition to a reduction in tumor size and extensive cystic changes, may indicate disease response[15].
In conclusion, we present here a rare case of gastric GIST with prominent calcification. To assess the mechanism and clinical relevance of calcification of GIST at presentation and following administration of imatinib, further accumulation of patients with pathological investigation would be required.
ACKNOWLEDGMENTS
The authors thank Mr. Eric Stewart for editing the manuscript.
Footnotes
Peer reviewer: Dr. Xiao-Yun Liao, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Room JF-208E, Boston, MA 02215, United States
S- Editor Gou SX L- Editor Rutherford A E- Editor Li JY
References
- 1.Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111–127. doi: 10.1007/s00428-010-0891-y. [DOI] [PubMed] [Google Scholar]
- 2.Machairas A, Karamitopoulou E, Tsapralis D, Karatzas T, Machairas N, Misiakos EP. Gastrointestinal stromal tumors (GISTs): an updated experience. Dig Dis Sci. 2010;55:3315–3327. doi: 10.1007/s10620-010-1360-9. [DOI] [PubMed] [Google Scholar]
- 3.Darnell A, Dalmau E, Pericay C, Musulén E, Martín J, Puig J, Malet A, Saigí E, Rey M. Gastrointestinal stromal tumors. Abdom Imaging. 2006;31:387–399. doi: 10.1007/s00261-004-0092-8. [DOI] [PubMed] [Google Scholar]
- 4.Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, Kotter E, Langer M. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003;13:1669–1678. doi: 10.1007/s00330-002-1803-6. [DOI] [PubMed] [Google Scholar]
- 5.Tateishi U, Hasegawa T, Satake M, Moriyama N. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr. 2003;27:792–798. doi: 10.1097/00004728-200309000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Kim HC, Lee JM, Kim KW, Park SH, Kim SH, Lee JY, Han JK, Choi BI. Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am J Roentgenol. 2004;183:893–898. doi: 10.2214/ajr.183.4.1830893. [DOI] [PubMed] [Google Scholar]
- 7.Sandrasegaran K, Rajesh A, Rushing DA, Rydberg J, Akisik FM, Henley JD. Gastrointestinal stromal tumors: CT and MRI findings. Eur Radiol. 2005;15:1407–1414. doi: 10.1007/s00330-005-2647-7. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida H, Mamada Y, Taniai N, Mizuguchi Y, Nakamura Y, Nomura T, Okuda T, Uchida E, Fukuda Y, Watanabe M, et al. Spurt bleeding from a calcificated gastrointestinal stromal tumor in the stomach. J Nippon Med Sch. 2005;72:304–307. doi: 10.1272/jnms.72.304. [DOI] [PubMed] [Google Scholar]
- 9.Rana R, Nikolaidis P, Miller F. Calcified gastrointestinal stromal tumor of the sigmoid colon mimicking inspissated barium on CT. AJR Am J Roentgenol. 2006;187:W322–W323. doi: 10.2214/AJR.06.0035. [DOI] [PubMed] [Google Scholar]
- 10.Ong K, Singaporewalla RM, Tan KB. Extensive calcification within a gastrointestinal stromal tumour: a potential diagnostic pitfall. Pathology. 2006;38:451–452. doi: 10.1080/00313020600924922. [DOI] [PubMed] [Google Scholar]
- 11.Testroote M, Hoornweg M, Rhemrev S. Rectal GIST presenting as a submucosal calculus. Dig Dis Sci. 2007;52:1047–1049. doi: 10.1007/s10620-006-9160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz RJ Jr, Vincenzi R, Ketzer BM, Cecilio AL, Cepeda LA. Spontaneous intratumoral bleeding and rupture of giant gastric stromal tumor (> 30 cm) in a young patient. World J Surg Oncol. 2008;6:76. doi: 10.1186/1477-7819-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu CC, Wu CC, Hwang JI, Wang J, Chang CS. Thick calcification from a GIST of the stomach penetrating into pericolic soft tissue--report of a case. World J Surg Oncol. 2011;9:45. doi: 10.1186/1477-7819-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichiishi E, Kogawa T, Takeda S, Yanagida K, Yoshikawa T, Kondo M. Eight cases of gastric tumors with calcification. Intern Med. 1995;34:1038–1042. doi: 10.2169/internalmedicine.34.1038. [DOI] [PubMed] [Google Scholar]
- 15.Warakaulle DR, Gleeson F. MDCT appearance of gastrointestinal stromal tumors after therapy with imatinib mesylate. AJR Am J Roentgenol. 2006;186:510–515. doi: 10.2214/AJR.04.1516. [DOI] [PubMed] [Google Scholar]
- 16.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 17.Berman J, O’Leary TJ. Gastrointestinal stromal tumor workshop. Hum Pathol. 2001;32:578–582. doi: 10.1053/hupa.2001.25484. [DOI] [PubMed] [Google Scholar]
- 18.Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–2020. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 19.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 20.Bechtold RE, Chen MY, Stanton CA, Savage PD, Levine EA. Cystic changes in hepatic and peritoneal metastases from gastrointestinal stromal tumors treated with Gleevec. Abdom Imaging. 2003;28:808–814. doi: 10.1007/s00261-003-0021-2. [DOI] [PubMed] [Google Scholar]


