Figure 1.
Generation of ΔPDZ-Prx Mice Expressing a Mutant Form of Periaxin Lacking the N-Terminal PDZ Domain
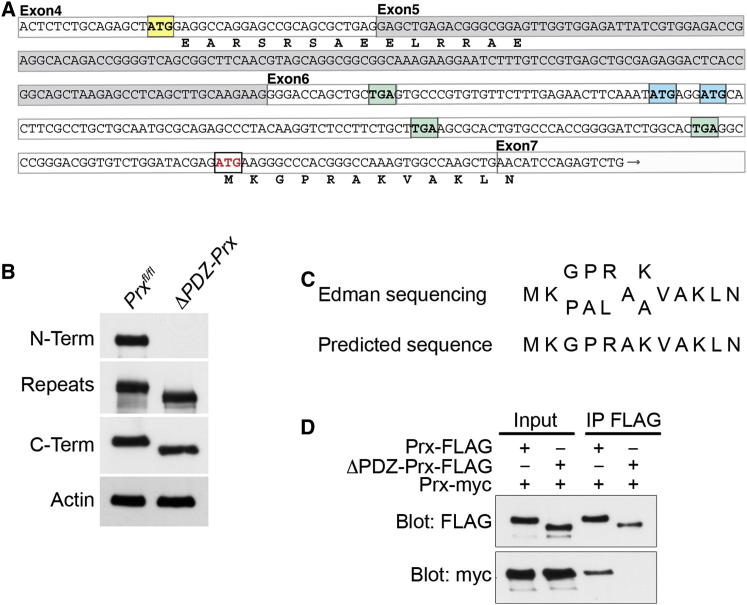
(A) The exon structure that encodes the N terminus of periaxin is shown with the normal initiation codon in exon 4 highlighted in yellow. Exon 5, which is deleted after Cre-mediated recombination, is outlined in gray, and the first in-frame stop codon in exon 6 is shown in green followed by two potential initiation codons in blue. These are followed by two in-frame stop codons in green, after which the putative initiation codon utilized in ΔPDZ-Prx mice is highlighted in red. The amino acid sequence recognized by the N-Term anti-periaxin antibody is shown (EARSRSAEELRRAE), as is the putative N-terminal amino acid of the ΔPDZ-Prx protein (MKGPRAKVAKLN).
(B) Western blot showing that an antibody raised against the peptide EARSRSAEELRRAE at the N terminus of wild-type periaxin (N-Term) does not recognize the ΔPDZ-Prx protein in extracts of sciatic nerves from 4-week-old mice, although the mutant protein reacts with two antibodies (Repeats and C-Term) that were raised against peptides encoded by exon 7. The shift to an increased mobility was also consistent with the mutant protein being slightly smaller than wild-type periaxin. γ-actin was the loading control.
(C) Although there was some ambiguity at four positions, sequential amino acid sequencing of the purified ΔPDZ-Prx protein from the N terminus by the Edman degradation technique for 12 rounds confirmed the new N terminus of the ΔPDZ-Prx protein depicted in (A).
(D) Coimmunoprecipitation from lysates of HEK293 cells transfected with cDNAs encoding myc-tagged wild-type periaxin with either FLAG-tagged wild-type periaxin or the myc-tagged mutant ΔPDZ-Prx showed that the mutant protein lacking the N-terminal PDZ domain did not interact with wild-type periaxin.