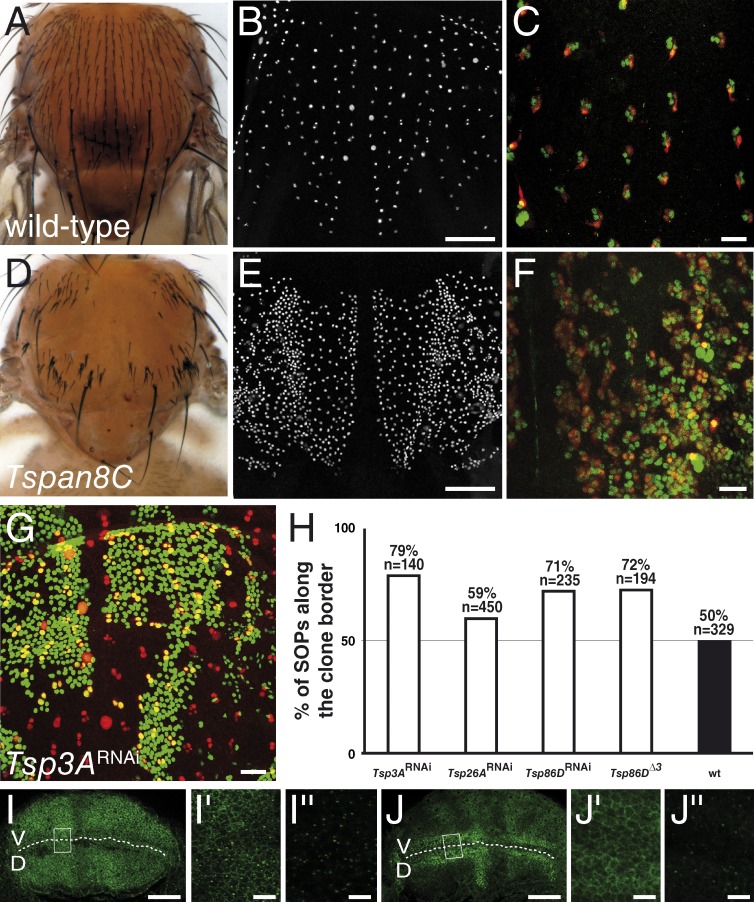
Figure 2.
Drosophila TspanC8 genes are required for Notch receptor signaling. (A–F) Wild-type pattern of sensory organs in adult flies (A) and of SOPs in 16 h after puparium formation (APF) pupae (B; positive for nuclear Senseless in white in B and E). At 22 h APF, each sense organ is composed of four cells (C and F; Cut in green). One of these four cells is a neuron (C and F; Elav in red). TspanC8 flies exhibited a bristle loss phenotype (D) associated with the specification of too many SOPs (E) and the transformation of external cells into internal cells, including neurons (F). TspanC8 corresponds here to the silencing of Tsp3A and Tsp26A by ap-GAL4 in a Tsp86D heterozygous background (see Table S1 for complete genotype). (G) Clones of cells silenced for Tsp3A were marked by nuclear GFP (green) in the notum of developing pupae. Competition for the SOP fate was studied by scoring the genotype (i.e., GFP) of SOPs (Senseless [Sens] in red) along clone borders. (H) Histogram showing the percentage of GFP-positive SOPs along clone borders for various genotypes (n is the number of scored SOPs). Control wild-type clones were also studied (wt). For each genotype, the distribution was significantly different from wild-type (χ2 test, P < 0.0001). (I–J′′) Wing imaginal discs stained for Notch (I–I′′) and Delta (J–J′′) from third instar larvae silenced for Tsp3A, Tsp26A, and Tsp86D using ap-Gal4 that directs transgene expression in dorsal cells (D; V is ventral; the DV boundary is indicated by a dashed line). I′, I′′, J′, and J′′ (I′ and J′: surface views; I′′ and J′′: −7 µm from the surface) show high magnification views of the area boxed in I and J, respectively. The silencing of the TspanC8 genes in D cells did not change the levels or distribution of Notch and Delta (compare with control V cells). Bars: (B and E) 100 µm; (C, F, G, I, and J) 25 µm; (I′′ and J′′) 5 µm.