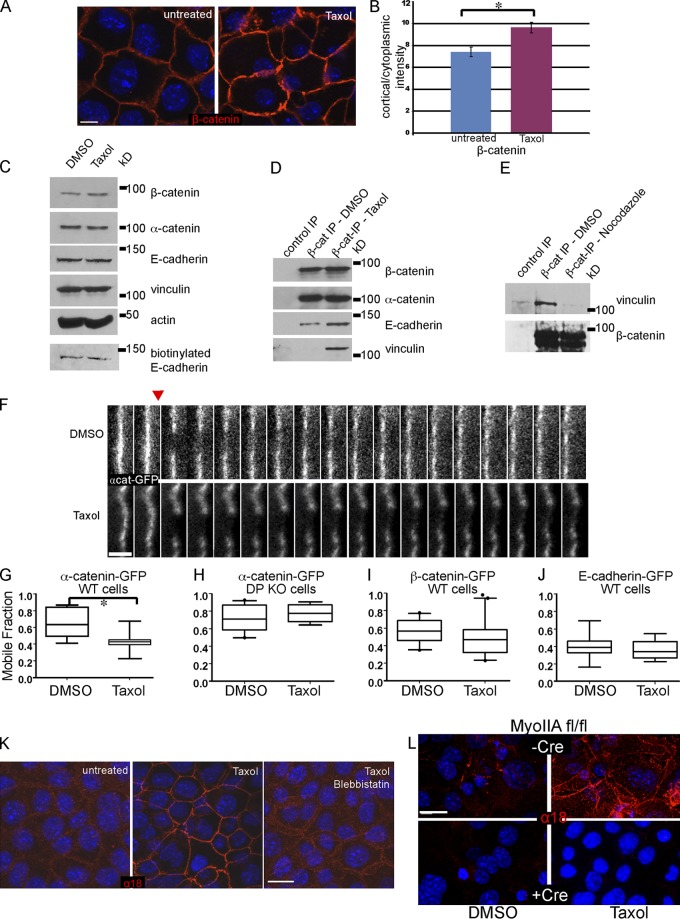
Figure 3.
Cortical microtubules induce engagement of adherens junctions. (A) Immunofluorescence analysis of β-catenin localization in control and taxol-treated cells, as indicated. (B) Quantitation of the cortical/cytoplasmic intensity measurements of β-catenin in control and taxol-treated cells. P = 0.014, n = 200 cells. (C) Western blots of adherens junction components in control and taxol-treated cell extracts, as indicated. The bottom panel represents exposed E-cadherin as identified by cell surface biotinylation. (D) Analysis of β-catenin immunoprecipitates from control and taxol-treated cultured cells. Proteins were subjected to Western blot analysis with E-cadherin, β-catenin, α-catenin, and vinculin antibodies. (E) Analysis of β-catenin immunoprecipitates from control and nocodazole-treated epidermal tissue extracts. Proteins were subjected to Western blot analysis with β-catenin and vinculin antibodies. (F) FRAP analysis of α-catenin-GFP in control and taxol-treated cells. Shown is a kymograph over 1 min. The red arrow indicates the time of photobleaching. Bar, 2 µm. (G–J) Quantitation of the mobile fraction of indicated adherens junction proteins in control and taxol-treated cells. All are in wild-type cells, except H, which is in desmoplakin-null cells. Boxes are 25–75% marks, whiskers are 5–95% marks. For G, P = 0.004; n = 8 for control and 11 for taxol treatment. (K) Immunofluorescence analysis of wild-type cells with the α18 antibody, which recognizes a tension-dependent conformation of α-catenin. Control cells, taxol-treated cells, and taxol plus blebbistatin-treated cells are shown, as indicated. (L) Immunofluorescence analysis of α18 in control and myosin IIA–null cells in the presence and absence of taxol, as indicated. Bars: (A, K, and L) 10 µm.