Abstract
A fungus detected from the importing seeds of Papaver rhoeas under plant quarantine inspection in Korea was identified as Brachycladium penicillatum Corda. It differed in morphological characteristics from a similar species, B. papaveris, which was known to form no macroconidiophores and no microsclerotia. Since the first interception in 2006, this fungus has frequently been found from importing seeds of Papaver spp. It was detected from 31 out of 282 seed consignments imported from 2006 to 2011. To prevent its introduction to Korea, the seed consignments infested by B. penicillatum were destroyed or reshipped.
Keywords: Brachycladium penicillatum, Dendryphion, Papaver, Plant quarantine, Seed-borne
Various kinds of seeds are imported into Korea from abroad. The amount of imported seeds has shown a continuous increase. Papaver spp. are species of flowering plants in the poppy family, Papaveraceae. Among them, growth and import of Papaver somniferum L. is prohibited in Korea, because it can be synthetically converted to the illicit drug heroin. However, some other Papaver spp. seeds have recently been imported from various countries for use as flowering plants.
Under the plant quarantine inspection, a fungus considered as Dendryphion species was initially detected from seeds of Papaver rhoeas in 2006. Sporulation of the fungus can be induced by use of the blotter method [1]. Three blotters were soaked in tap water and placed in Petri dishes. Fifty seeds were placed on wetted blotters in Petri dishes. After placement of the seeds, they were incubated at 22℃ with alternating 12-hr cycles of fluorescent light and darkness. Sporulation was observed from the third day of incubation and was well recognized at the fifth day. A compound microscope was used for observation of detailed structures of the fungus. Pieces of fungal tissues were transferred from seeds to a microscopic slide and then mounted in lactic acid.
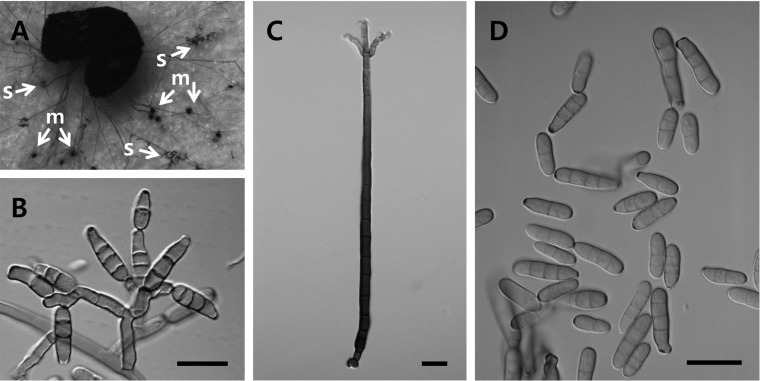
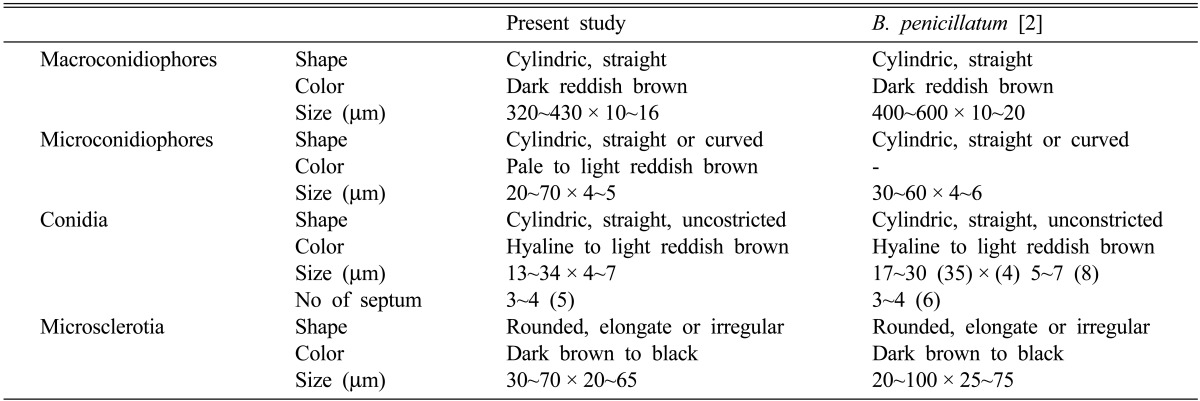
It formed macroconidiophores and microconidiophores (Fig. 1B and 1C). Macroconidiophores were erect, cylindric, straight, and simple, and then branched 2~3 times near the apex, and were dark reddish brown, measuring 320~430 × 10~16 µm in size. Microconidiophores were cylindric, straight or curved, pale to light reddish brown, measuring 20~70 × 4~5 µm in size. Conidia were solitary or in chains, cylindric, straight, hyaline to light reddish brown, unconstricted, smooth, and measured 13~34 × 4~7 µm in size, with 3~4 (5) septa (Fig. 1D). Microsclerotia were additionally observed on the seeds and blotter at around the fifth day after incubation (Fig. 1A). They were rounded, elongate or of irregular shape, dark brown to black, and measured 30~70 × 20~65 µm in size. Based on these morphological characteristics, this fungus was identified as Brachycladium penicillatum Corda.
Fig. 1.
Morphological features of Brachycaldium penicillatum from seeds of Papaver spp. A, Microrsclerotia (m) and sporulation (s) produced from seeds on blotter; B, Microconidiophores forming solitary or in chains of conidia; C, Macroconidiophore; D, Conidia (scale bars = 20 µm).
These features were similar to those described previously by Inderbitzin et al. [2] (Table 1). B. penicillatum was formerly known as Dendryphion penicillatum (Corda) Fr., the conidial state of Pleospora papaveracea [3, 4]. Shoemaker [5] suggested that Pleospora papaveracea contained one sexual species with the asexual state Dendryphion penicillatum (Corda) Fr. On the other hand, Farr et al. [6] found two species among the isolates of P. papaveracea; one of these formed a sexual state, and the other did not. They concluded that the correct asexual state of P. papaveracea was Dendryphiella sp., and that D. penicillatum was a distinct asexual species without known sexual state. However, as a revision, Inderbitzin et al. [2] reported that B. penicillatum corresponds to D. penicillatum, and Brachycladium papaveris, a new combination, corresponds to Dendryphiella sp. based on the molecular and morphological analysis.
Table 1.
Morphological characteristics of Brachycladium penicillatum detected from seeds of Papaver rhoeas
The fungus intercepted in this study was easily differentiated from B. papaveris. Morphological features of B. penicillatum resemble those of B. papaveris. However, presentation of microsclerotia is observed in B. penicillatum but not in B. papaveris. B. penicillatum forms macroconidiophores but B. papaveris does not [2].
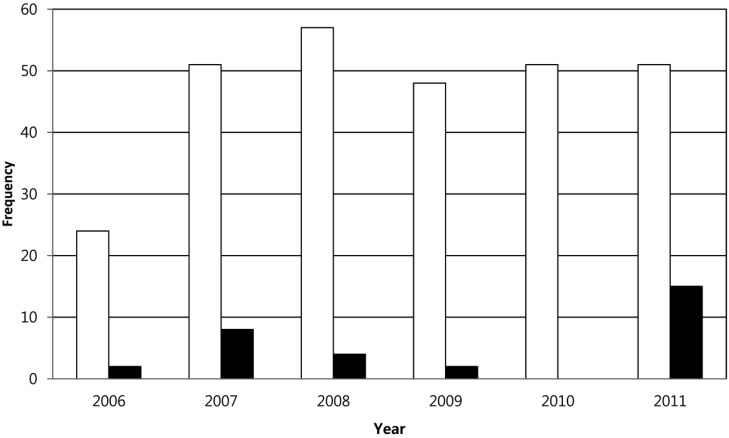
After the first interception in 2006, B. penicillatum was frequently found from importing seeds. This fungus exhibits host specific characteristics. It was detected only from seeds of Papaver spp., including P. nudicaule, P. rhoeas, and P. orientale. On the other hand, as the import is increasing, the frequency of interception has also increased (Fig. 2). It was intercepted from 31 out of 282 seed consignments imported from 2006 to 2011. Distribution of B. penicillatum has been reported in Afghanistan, Australia, Austria, Azerbaijan, Colombia, Germany, Hungry, India, Iran, Japan, Nepal, Pakistan, Poland, Romania, South Africa, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, USA, Venezuela, and Zambia [4, 7]. During the last six years, B. penicillatum has been detected during plant quarantine inspection from seeds imported from Canada, China, Germany, Netherlands, New Zealand, Sweden, United Kingdom, and USA. Therefore, the fungus is also considered to be distributed in Canada, China, Netherlands, and New Zealand.
Fig. 2.
Frequency of interception of Brachycladium penicillatum from seeds of Papaver spp. under quarantine inspection from 2006 to 2011. White and black bars indicate frequency of importation of Papaver spp. seeds and frequency of interception, respectively.
B. penicillatum is a quarantine pest of Korea, which causes leaf blight disease of Papaver spp. It has even been studied for its potential as a mycoherbicide against Papaver somniferum [8, 9]. Existence of the fungus in Korea has not been reported [10]. Seeds infested by exotic seed-borne fungi may cause progressive development of disease in the field, which can result in reduction of yield and quality. The seed consignments infested by B. penicillatum were destroyed or reshipped in order to prevent its introduction to Korea. Intensive plant quarantine is necessary in order to prevent introduction of the invasive fungal pathogen into Korea.
References
- 1.Agarwal VK, Sinclair JB. Principles of seed pathology. 2nd ed. Boca Raton: CRC Press; 1997. [Google Scholar]
- 2.Inderbitzin P, Shoemaker RA, O'Neill NR, Turgeon BG, Berbee ML. Systematics and mating systems of two fungal pathogens of opium poppy: the heterothallic Crivellia papaveracea with a Brachycladium penicillatum asexual state and a homothallic species with a Brachycladium papaveris asexual state. Can J Bot. 2006;84:1304–1326. [Google Scholar]
- 3.Ellis MB. Dematiaceous hyphomycetes. Surrey: Commonwealth Mycological Institute; 1971. [Google Scholar]
- 4.Sivanesan A, Holliday P. Pleospora papaveracea. CMI Descr Pathog Fungi Bact. 1982;730:1–2. [Google Scholar]
- 5.Shoemaker RA. Type studies of Pleospora calvescens, Pleospora papaveracea, and some allied species. Can J Bot. 1968;46:1143–1150. [Google Scholar]
- 6.Farr DF, O'Neill NR, van Berkum PB. Morphological and molecular studies on Dendryphion penicillatum and Pleospora papaveracea, pathogens of Papaver somniferum. Mycologia. 2000;92:145–153. [Google Scholar]
- 7.Farr DF, Rossman AY. Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA [Internet] Beltsville: Systematic Mycology and Microbiology Laboratory, ARS, USDA; [cited 2012 Jul 18]. Available from http://nt.ars-grin.gov/fungaldatabases/ [Google Scholar]
- 8.Bailey BA, Apel-Birkhold PC, O'Neill NR, Plaskowitz J, Alavi S, Jennings JC, Anderson JD. Evaluation of infection processes and resulting disease caused by Dendryphion penicillatum and Pleospora papaveracea on Papaver somniferum. Phytopathology. 2000;90:699–709. doi: 10.1094/PHYTO.2000.90.7.699. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill NR, Jennings JC, Bailey BA, Farr DF. Dendryphion penicillatum and Pleospora papaveracea, destructive seedborne pathogens and potential mycoherbicides for Papaver somniferum. Phytopathology. 2000;90:691–698. doi: 10.1094/PHYTO.2000.90.7.691. [DOI] [PubMed] [Google Scholar]
- 10.Korean Society of Plant Pathology. List of plant diseases in Korea. 5th ed. Suwon: Korean Society of Plant Pathology; 2009. [Google Scholar]