Abstract
Ever since the interest in organic environmental contaminants first emerged 50 years ago, there has been a need to present discussion of such chemicals and their transformation products using simple abbreviations so as to avoid the repetitive use of long chemical names. As the number of chemicals of concern has increased, the number of abbreviations has also increased dramatically, sometimes resulting in the use of different abbreviations for the same chemical. In this article, we propose abbreviations for flame retardants (FRs) substituted with bromine or chlorine atoms or including a functional group containing phosphorus, i.e. BFRs, CFRs and PFRs, respectively. Due to the large number of halogenated and organophosphorus FRs, it has become increasingly important to develop a strategy for abbreviating the chemical names of FRs. In this paper, a two step procedure is proposed for deriving practical abbreviations (PRABs) for the chemicals discussed. In the first step, structural abbreviations (STABs) are developed using specific STAB criteria based on the FR structure. However, since several of the derived STABs are complicated and long, we propose instead the use of PRABs. These are, commonly, an extract of the most essential part of the STAB, while also considering abbreviations previously used in the literature. We indicate how these can be used to develop an abbreviation that can be generally accepted by scientists and other professionals involved in FR related work. Tables with PRABs and STABs for BFRs, CFRs and PFRs are presented, including CAS (Chemical Abstract Service) numbers, notes of abbreviations that have been used previously, CA (Chemical Abstract) name, common names and trade names, as well as some fundamental physico-chemical constants.
Keywords: Brominated flame retardants, Chlorinated flame retardants, Phosphorus flame retardants, Nomenclature, Abbreviations, Physico-chemical properties
1. Introduction
Even though the history of flame retardants (FRs) dates back thousands of years (Hindersinn, 1990), it is the recent developments, and in particular the use of organic FRs, that is of current concern. Two of the major groups of these FRs are (i) halogenated FRs that may be divided into brominated and chlorinated flame retardants (BFRs and CFRs, respectively), and (ii) phosphorus-containing flame retardants (PFRs). The BFRs, CFRs and PFRs cover the major proportion of organic FRs, although some FRs contain neither halogen nor phosphorus atoms (e.g. melamine, 1,3,5-triazine-2,4,6-triamine). FRs are incorporated as either additive or reactive ingredients, with the aim of increasing the fire resistance of materials. Hence, reactive FRs are incorporated into the oligomers or polymers being manufactured, while additive FRs are molded within the material to be flame retarded.
Some countries or states have rather unique regulations requiring furniture and electrical equipment to meet specific flammability tests, e.g. in the UK and Ireland (Arcadis EBRC, 2011); and in California in the USA (State of California, 2000). However, there is growing evidence that these regulations may not offer the protection that was first intended (Babrauskas et al., 2012; DiGangi et al., 2010). Also, there is a growing body of knowledge which is raising concerns about these chemicals in relation to their persistence, bioaccumulation, toxicity and long range transport. The ‘San Antonio Statement’ (DiGangi et al., 2010) sets the scene as to why this topic is of major concern to the global society. The FR area is complex, with numerous individual chemicals comprising the BFRs, CFRs and PFRs. This highlights the need for a common vocabulary amongst scientists and others to be used when addressing these chemicals in order to avoid confusion.
1.1. History of organic flame retardants
Polychlorinated biphenyls (PCBs) were manufactured and applied as FRs from the late 1920s until the mid-1980s, although PCBs were also used in a multitude of other applications, particularly in electrical equipment. Other chlorinated compounds came into use as FR, probably from the 1960s onwards, sometimes also including a phosphate group, such as the tris–(2,3-dichloropropyl)phosphate (TDCPP) and tris–(1,3-dichloro-iso-propyl)phosphate (TDCIPP) (Gold et al., 1978). The brominated analog of the former compound, tris–(2,3-dibromopropyl)phosphate (TDBPP) made the headlines in the 1970s due to its use in children’s pajamas (Blum et al., 1978). In the beginning of the 1970s, an increasing number of BFRs, e.g. polybrominated biphenyls (PBBs) and polybrominated diphenyl ethers (PBDEs), came to the market. In 1997, the World Health Organization tried to list all major FRs, also including any inorganic chemicals used in that role (WHO/IPCS, 1997). Pijnenburg et al. (1995) made the first review of BFRs, including what was known of their analysis, toxicity and environmental occurrence, and numerous other reviews and/or assessment documents have been published since then (e.g. Bergman, 2005; Birnbaum and Staskal, 2004; D’Silva et al., 2004; de Boer et al., 2000; de Wit, 2002; Law et al., 2003). Among the most recent documents concerning BFRs are five published opinions from the European Food Safety Authority (EFSA) on PBBs (EFSA, 2010), PBDEs (EFSA, 2011a), hexabromocyclododecanes (HBCDDs) (EFSA, 2011b), TBBPA and its derivatives (EFSA, 2011c) and also an opinion concerning other phenolic BFRs and their derivatives (EFSA, 2012). EFSA is presently also preparing an opinion on emerging and novel BFRs, for publication in 2012. In 2011, a book on BFRs was published which covered a multitude of issues relating to BFRs (Eljarrat and Barceleó, 2011). Other major reviews of BFRs from 2005 onwards include Covaci et al. (2006, 2009, 2011), Law et al. (2006, 2008). A review on PFRs was recently published (van der Veen and de Boer, 2012) while, among the CFRs, only the Dechloranes have been comprehensively reviewed to date (Sverko et al., 2011).
The BFRs most commonly used today are tetrabromobisphenol A (TBBPA), decabromodiphenyl ether (DecaBDE) and HBCDD (also sometimes referred to as HBCD). Due to EU legislative measures and the inclusion of PentaBDE and OctaBDE among the Stockholm Convention POPs, there are now changes in the production and use of PBDEs, HBCDDs and many other BFRs, including some which are being used as replacements for now restricted formulations. DecaBDE is subjected to use restrictions according to the RoHS directive (Directive 2002/95/EC (OJ, 2003)) after the European Court of Justice decision from 2008 (OJ, 2008). However, these changes cannot be documented adequately as the producers do not make production figures available, regardless of where the chemicals are manufactured. Similarly, there is little information available on the current applications in which these compounds are being used. The situation is similar also for production and use of CFRs and PFRs.
It is safe to say that the use of BFRs has increased dramatically since the 1970s and their cumulative current production volume exceeds 200,000 t per year, based on available information (personal communication, V. Steukers, Albemarle, 2008; references in Eljarrat and Barceleó, 2011). Volumes of CFRs seem to be higher since, in 2007, the production of polychlorinated alkanes (PCAs) (also known as chlorinated paraffins (CPs)) amounted to up to 600,000 t per year, in China alone (Fiedler, 2010). These compounds are not solely used as flame retardants, however, and have a number of other applications (Nicholls et al., 2001). The worldwide production volume of PFRs in 2004 was slightly above 200,000 t per year (EFRA, 2007).
Due to the increased regulatory interest in and restrictions on PBDEs and HBCDD, alternative FRs are now being used in their place. It is, as shown below, difficult even to list those BFRs currently being offered for sale in the market. In the present document, we are therefore presenting all BFRs, CFRs and PFRs that have been proposed to date for use as FRs. Several FRs have only recently been detected in the environment, even though they may have been in use for some time, e.g. Dechlorane Plus (Sverko et al., 2011). The analysis, environmental fate and behavior of novel BFRs have been reviewed (Covaci et al., 2011; Papachlimitzou et al., 2012) and they are presently under review by EFSA. A suite of FRs has also been reported as present in materials and products taken recently from the Swiss retail market (Zennegg, 2011). In addition, other types of compounds are also used as FRs in a variety of applications, notably PFRs. Regarding the present use of CFRs, less has been published to date, even though some new chemicals have now been identified as CFRs. These are mainly related to the family of “Dechloranes” (Sverko et al., 2011) as further discussed below.
1.2. Aims
As the number of compounds in use as FRs, and for which environmental data are being reported increases, there is a pressing need to harmonize abbreviations by which these compounds can be described in the literature (for example, using TBBPA and PBDEs as described above, and BDE47 for 2,2′,4,4′-tetrabromodiphenyl ether), with the aim of preventing future confusion. Unfortunately, a rather large number of abbreviations, for the less known FRs, are currently being used without any coordination. Following a request made at the BFR Symposium 2010 in Kyoto, we have now prepared a document which aims to promote improved harmonization, based on a set of criteria, of unique and practical abbreviations to be used for all BFRs, CFRs and PFRs identified to date. In this paper, we provide information relating to halogenated FRs and PFRs, including common, trade and systematic names, CAS numbers, physicochemical properties where known, together with recommended structured abbreviations (STABs) and practical abbreviations (PRABs). Also some general comments and suggestions are given with the aim of simplifying the abbreviation of the full chemical names of BFRs, CFRs and PFRs.
2. Methodology
All compounds listed were retrieved by reviewing the scientific literature for BFRs, CFRs and PFRs. Documents of particular use for identifying BFRs and CFRs were: WHO/IPCS (1994, 1995), WHO/IPCS (1997), Örn and Bergman (2004), Andersson et al. (2006); Harju et al. (2009), Letcher et al. (2009), Covaci et al. (2011), de Wit et al. (2011),Sverko et al. (2011); and for PFRs: van der Veen and de Boer (2012).
The compounds are presented in three separate groups (BFRs, CFRs and PFRs) and then listed in molecular mass order within each subgroup. The sub-grouping is given below. We have chosen to list FRs holding, for example, both a phosphorus group and a halogen substituent, in each of the groups to which they belong, i.e. a BFR with a chlorine substituent is also listed in the table containing CFRs (Table 3); a PFR containing bromine substituents is also listed as a BFR. This means that some of the chemicals are listed twice.
Table 3.
Practical abbreviation (PRAB, in bold) for chlorine containing flame retardants, together with structured abbreviations (STAB; plain text) are presented. The table also includes some basic physicochemical constants calculated using ACD/Labs Software V11.02. The STABs are constructed as described under “Methodology”, incl. Table 1.
| CAS number |
PRABs STABs |
Previously used abbreviations |
CA name | Common and trade names | Additive or reactive BFR |
MW | Log Kow |
Koc | pKa | Vapor pressure (Pa) |
|---|---|---|---|---|---|---|---|---|---|---|
| 117-08-8 |
TECP-Anh TeCPht-Anh |

|
Tetrachlorophthalic anhydride Phthalic anhydride, tetrachloro- (6CI,8CI) 1,3-Dioxy-4,5,6,7-tetrachloroisobenzofuran 3,4,5,6-Tetrachlorophthalic anhydride NSC 1484 Niagathal Tetrachlorophthalic acid anhydride |
R | 285.9 | 3.5 | 1900 | na | 1.41E-03 | |
| 39569-21-6 |
TBCT TeBCMeBza |
TBoCT |

|
2,3,4,5-tetrabromo-6-chloromethylbenzene 2,3,4,5-Tetrabromo-6-chlorotoluene Tetrabromo-o-chlorotoluene |
A | 442.17 | 6.29 | 62,800 | na | 1.72E-03 |
| 77-47-4 |
HCCPD HxCcPe(dien) |

|
Hexachlorocyclopentadiene Cyclopentadiene, hexachloro- (7CI) 1,2,3,4,5,5-Hexachloro-1,3-cyclopentadiene 1,2,3,4,5,5-Hexachlorocyclopentadiene C 56 Graphlox HRS 1655 Hexachloro-1,3-cyclopentadiene NSC 9235 Perchlorocyclopentadiene |
A | ||||||
| 115-27-5 |
HCBCH-DCAnh HxCbcHte-DiCaAnh |

|
1,4,5,6,7,7-Hexachlorobicyclo[2.2.1]hept-5-ene- 2,3-dicarboxylic anhydride 5-Norbornene-2,3-dicarboxylic anhydride, 1,4,5,6,7,7- hexachloro- (6CI,8CI) Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride,1, 4,5,6,7,7-hexachloro- (5CI) 1,4,5,6,7,7-Hexachloro-5-bicyclo[2.2.1]heptene-2,3- dicarboxylic anhydride 1,4,5,6,7,7-Hexachloro-5-norbornene-2,3-dicarboxylic anhydride 1,4,5,6,7,7-Hexachlorobicyclo[2.2.1]-5-heptene-2,3- dicarboxylic acid anhydride 2,3-Dicarboxy-1,4,5,6,7,7- hexachlorobicyclo[2.2.1]hept-5-ene anhydride 3,4,5,6,7,7-Hexachloro-1,2,3,6-tetrahydro-3,6- endomethylenephthalic anhydride Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid, 1,4,5,6,7,7-hexachloro-, anhydride Chloran 542 Chlorendic anhydride HET Anhydride Hexachloro-5-norbornene-2,3-dicarboxylic anhydride Hexachloroendomethylene tetrahydrophthalic anhydride Kayahard CLA NSC 22229 endo-1,4,5,6,7,7-Hexachloro-1,2,3,4-tetrahydro- 5-norbornene-2,3-dicarboxylic acid anhydride |
R | 370.83 | 1.33 | 126 | na | 9.79E-07 | |
| 115-28-6 |
HCBCH-DCA HxCbcHte-DiCaA |
HET acid |

|
1,4,5,6,7,7-Hexachlorobicyclo-(2,2,1)hept-5-en- 2,3-dicarboxylic acid 5-Norbornene-2,3-dicarboxylic acid, 1,4,5,6,7,7- hexachloro-(6CI,8CI); 1,4,5,6,7,7-Hexachloro-5-norbornene-2,3- dicarboxylic acid 1,4,5,6,7,7-Hexachlorodicyclo-(2.2.1)-5-heptene-2,3- dicarboxylic acid 2H,3H-Hexachlorobicyclo[2.2.1]hept-5-ene-2,3- dicarboxylic acid Chlorendic acid HET acid Hexachloroendomethylenetetrahydrophthalic acid NSC 22231 NSC 41876 |
R | 388.84 | 4.6 | pH-dep. | 1.26 | 4.49E-08 |
| 51936-55-1 |
DBHCTD DiBHxCTrcTrDenb |
HCDBCO |

|
5,6-Dibromo-1,10,11,12,13,13-hexachloro-11- tricyclo[8.2.1.02,9]tridecene 7,8-Dibromo-1,2,3,4,11,11-hexachloro-1,4, 4a,5,6,7,8,9,10,10a-decahydro-1,4-methanobenzo [8]annulene Saytex BC 26 Citex BC 26 |
A | 540.76 | 7.62 | 332E+05 | na | 8.27E-07 |
| 2385-85-5 |
MIREX Per-CPecDe |
MIREX |

|
Perchloropentacyclodecane Perchloropentacyclo[5.2.1.02,6.03,9.05,8]decane 1,3,4-Metheno-1H-cyclobuta[cd]pentalene, dodecachlorooctahydro- (7CI) 1,3-Cyclopentadiene, 1,2,3,4,5,5-hexachloro-, dimer Dechlorane Dechlorane 4070 Dodecachlor Dodecachlorooctahydro-1,3,4-metheno- 2Hcyclobuta[cd]pentalene Dodecaclor ENT 25719 GC 1283 Hexachlorocyclopentadiene dimer Mirex NSC 124102 NSC 26107 NSC 37656 Paramex Perchlorodihomocubane Perchloropentacyclo[5.3.0.02,6.03,9.04,8]decane |
545.54 | 7.11 | 1.75E+ 05 | na | 1.01E-04 | |
| 31107-44-5 |
DDC-DBF DDC-DiMeDiBzFb |
Dec 602 |

|
1,2,3,4,6,7,8,9,10,10,11,11-Dodecachloro-1, 4,4a,5a,6,9,9a,9b-octahydro-1,4:6,9- dimethanodibenzofuran Dechlorane 602 |
A | 613.62 | 83 | 7.78E+ 05 | na | 1.48E-09 |
| 13560-89-9 |
DDC-CO DDCDiMeDiBzcOb |
DP |

|
Dodecachlorodimethanodibenzocyclooctane 1,2,3,4,7,8,9,10,13,13,14,14-Dodecachloro- 1,4,4a,5,6,6a,7,10,10a,11,12,12a-dodecahydro- 1,4:7,10-dimethanodibenzo[a,e]cyclooctane Bis(hexachlorocyclopentadieno)cyclooctane Dechloran A Dechlorane Plus Dechlorane Plus 1000 Dechlorane Plus 25 Dechlorane Plus 2520 Dechlorane Plus 35 Dechlorane Plus 515 Dodecachlorododecahydrodimethanodibenzocyclooctane Dodecachlorododecahydrodimethanodibenzocyclooctene |
A | 653.72 | 10.12 | 7.67E+ 06 | na | 1.37E-11 |
| 13560-92-4 |
DDC-Ant DDC-TrMeAntb |
Dec 603 |

|
1,2,3,4,5,6,7,8,12,12,13,13-Dodecachloro-1,4,4a,5,8,8a,9,9a, 10,10a-decahydro-1,4:5,8:9,10-Trimethanoanthracene |
A | 637.68 | 9.14 | 2.24E+ 06 | na | 9.16E-10 |
| 34571-16-9 |
HCTBPH HxCTeBPhbcHen |
Dec 604 |

|
1,2,3,4,7,7-Hexachloro-5-(2,3,4,5-tetrabromophenyl)- Bicyclo[2.2.1]hept-2-ene 2-Norbornene, 1,2,3,4,7,7-hexachloro-5- (tetrabromophenyl)-(8CI) 5-(Tetrabromophenyl)-1,2,3,4,7,7-hexachloro- 2-norbornene Dechlorane 604 Component A Hexachlorocyclopentadiene-tetrabromostyrene adduct Dechlorane 604 |
A | 692.5 | 10.24 | 8.86E+ 06 | na | 1.61E-08 |
| See # | SCCPc | SCCP | Alkanes, C10-13, chloro CxH(2x–y + 2)Cly |
Short-chained chlorinated paraffins C10-13 chloro alkanes Chloro alkanes C10-13 Hordalub 500 PCA 60 PCA 70 Witaclor 149 Witaclor 171P sPCA 60 |
A | na | na | na | na | na |
| See# | MCCPc | MCCP | Alkanes, C14-17, chloro CxH(2x–y + 2)Cly |
Medium-chained chlorinated paraffins | A | na | na | na | na | na |
| See# | LCCPc | LCCP | Alkanes, C18-30, chloro CxH(2x–y + 2)Cly |
Long-chained chlorinated paraffins | A | na | na | na | na | na |
| 115-96-8 |
TCEP tCEtP |
TCEP |

|
Tris(chloroethyl) phosphate 3CF Amgard TCEP CEF CLP Celluflex CEF Disflamoll TCA Fyrol CEF Fyrol CF Genomoll P NSC 3213 Niax 3CF Niax Flame Retardant 3CF TCEP Tri(2-chloroethyl) phosphate Tri(chloroethyl) phosphate Tri(ß-chloroethyl) phosphate Tris(2-chloroethyl) orthophosphate Tris(2-chloroethyl) phosphate Tris(ß-chloroethyl) phosphate |
A | 285.49 | 1.47 | 150 | na | 1.44E-02 |
| 78-43-3 |
TDCPP tDiCPrP |
TDCPP |

|
Tris(2,3-dichloropropyl) phosphate 1-Propanol, 2,3-dichloro-, phosphate (3:1) (8CI,9CI) Celluflex FR 2 Fyrol 32B Tri(2,3-dichloropropyl) phosphate Tris(2,3-dichloro-n-propyl) phosphate |
A | 430.9 | 2.98 | 998 | na | 8.67E-07 |
| 13674-87-8 |
TDCIPP tDiCiPrP |
TDCIPP |

|
Tris(1,3-dichloroisopropyl) phosphate 3PC-R Antiblaze 195 CRP CRP (fireproofing agent) FR 10 FR 10 (phosphate) Fyrol FR 2 PF 38 PF 38/3 TDCPP Tris(1,3-dichloro-2-propyl) phosphate Tris(1-chloromethyl-2-chloroethyl)phosphate Tris[2-chloro-1-(chloromethyl)ethyl] phosphate |
A | 430.9 | 3.27 | 1440 | na | 5.43E-06 |
| 38051-10-4 |
BCMP-BCEP bCMePrDiOHbbCEtP |

|
2,2-Bis(chloromethyl)-1,3-propanediol bis[bis(2- chloroethyl)phosphate] Phosphoric acid, 2,2-bis(chloromethyl)-1,3-propanediyl tetrakis(2-chloroethyl) ester (9CI) Phosphoric acid, bis(2-chloroethyl) ester, diester with 2,2- bis(chloromethyl)-1,3-propanediol (7CI) 2,2-Bis(chloromethyl)-1,3-propanediol 2-chloroethanol phosphate (1:4:2) 2,2-Bis(chloromethyl)-1,3-propanediyl bis[bis(2-chloroethyl)phosphate] Amgard V 6 Antiblaze 100 Antiblaze AB 100 Antiblaze V 6 Phosgard 2XC20 |
A | 582.99 | 2.52 | 557 | na | 1.61E-12 |
It is possible to add the positions for the chlorine substituents in front of the abbreviation.
Some structural element is left out in the proposed STAB to minimize the complexity thereof.
Well established abbreviation. No change is proposed.
CAS numbers for SCCP, MCCP and LCCP: 61788-76-9, 63449-39-8, 68920-70-7,71011-12-6, 84082-38-2, 84776-07-8, 84776-06-7,85049-26-9, 85422-92-0, 85535-85-9, 85535-84-8,85535-86-0, 85536-22-7, 85681-73-8,97553-43-0, 97659-46-6,106232-86-4, 106232-85-3, 108171-27-3,108171-26-2.
One further goal of the systematic work presented herein is to enable us to treat functional groups in chemicals in a similar way, which could also be applied for hitherto unknown BFRs, CFRs, and PFRs that may be identified as commercial products in the future. This may be exemplified by the way in which we handle ether and ester functional groups when structured abbreviations are made. Allyl ethers of e.g. 2,4,6-tribromophenol and TBBPA are handled by naming the phenol entity first and then introducing one or two ether function-alities, the latter denoted “bis” (b), to give the STABs: TrBPh-AE and TBBPA-bAE, respectively. Other ethers are treated similarly, with the aryl group first and with the alkyl ether group linked to the word “ether”. In order to minimize confusion, we propose the use of a set of standardized short forms for major parts of a molecule (or their name). The criteria for constructing the abbreviations are given below and in Table 1. The STABs of all BFRs, CFRs and PFRs are listed in plain letters under the PRABs of the same compound, presented in bold letters (Tables 2–4).
Table 1.
Abbreviations of functional groups or corresponding entities in a molecule to be applied when constructing structured abbreviations (STABs).
| Letter | Group | Letter | Group | Letter | Group |
|---|---|---|---|---|---|
| A | Allyl | Ac | Acetate | Acr | Acrylate |
| AE | Allyl ether | Anh | Anhydride | Ant | Anthracene |
| B | Bromine | Bn | Benzyl | BnB | Benzylbromide |
| BnC | Benzylchloride | Bu | Butane or butyl | BuO | Butoxy |
| Bz | Benzo or benzene | Bzo | Benzoate | ||
| C | Chlorine | CaA | Carboxylic acid | ||
| DD | Dodecane or dodecyl | De | Decane or decyl | Den | Decen |
| E | Ether | Et | Ethane or ethyl | EtO | Ethoxy |
| F | Furan | ||||
| GE | Glycidyl ether | ||||
| H | Hexane or hexyl | Ht | Heptane or heptyl | Hte | Hepten |
| Im | Imide | In | Indane | ||
| Me | Methane or methyl | ||||
| N | Nonane or nonyl | ||||
| O | Octane or octyl | OH | Hydroxyl | OPO | Oxaphosphorinoxide |
| P | Poly – if first in abbreviation Phosphate – if last in abbreviation |
Ph | Phenol or phenyl | PhO | Phenoxy |
| Pht | Phthalate | Pr | Propane or propyl | Prt | Propionate |
| Pt | Pentane or pentyl | ||||
| Re | Resorcinol | ||||
| Sty | Styrene | ||||
| T | Toluene | Taz | Triazine | Tazto | Triazine-trione |
Table 2.
Practical abbreviation (PRAB, in bold) for bromine containing flame retardants, together with structured abbreviations (STAB; plain text) are presented. The table also includes some basic physicochemical constants calculated using ACD/Labs Software V11.02. The STABs are constructed as described under “Methodology”, incl. Table 1.
| CAS number |
PRABs STABs |
Previously used abbreviations |
CA name and structure | Common and trade names | Additive or reactive BFR |
MW | Log Kow |
Koc | pKa | Vapor pressure (Pa) |
|---|---|---|---|---|---|---|---|---|---|---|
| 615-58-7 |
DBP DiBPha |
2,4-BrPh DBP 24DBP |

|
2,4-Dibromophenol NSC 5723 NSC 6213 |
A/R | 251.9 | 3.47 | pH-dep | 7.86 | 3.65E + 00 |
| 31780-26-4 |
DBS DiBStya |
DBS DBrsty |

|
Dibromostyrene Styrene, ar,ar-dibromo- (8CI) Flame Cut 310K |
A/R | 261.94 | na | na | na | na |
| 118-79-6 |
TBP TrBPha |
2,4,6BrPh 246TBP 2,4,6-TBP TBP |

|
2,4,6-Tribromophenol 1,3,5-Tribromo-2-hydroxybenzene Bromkal Pur 3 Bromol Flammex 3BP NSC 2136 PH 73 |
A/R | 330.8 | 4.4 | pH-dep. | 6.34±0.23 | 2.00E-01 |
| 3278-89-5 |
TBP-AE TrBPh-AEa |
ATE TBrPhAE |

|
2,4,6-Tribromophenyl allyl ether Benzene, 1,3,5-tribromo-2-(2-propenyloxy)- (9CI) Allyl 2,4,6-tribromophenyl ether Ether, allyl 2,4,6-tribromophenyl (7CI,8CI) Pyroguard FR 100 NSC 35767 2-(allyloxy)-1,3,5-tribromobenzene Bromkal 64-3AE; PHE-65 |
A/R | 370.8 | 5.04 | 13,100 | na | 2.40E-02 |
| 23488-38-2 |
TBX TeBDiMeBza |
TBX p-TBX |

|
1,2,4,5-Tetrabromo-3,6-dimethylbenzene 2.3.5.6-Tetrabromo-p-xylene 1,4-Dimethyltetrabromobenzene 2,3,5,6-Tetrabromo-1,4-dimethylbenzene |
A | 421.75 | 6.2 | 56,100 | na | 5.80E-03 |
| 39569-21-6 |
TBCT TeBCT Tea |
TBCT TBoCT |

|
2,3,4,5-Tetrabromo-6-chlorotoluene Tetrabromo-o-chlorotoluene 2,3,4,5-tetrabromo-6-chloromethylbenzene |
A | 442.17 | 6.29 | 62,800 | na | 1.72E-03 |
| 632-79-1 |
TEBP-Anh TeBPht-Anh |
TBPA |

|
3.4.5.6-Tetrabromophthalic anhydride Phthalic anhydride, tetrabromo- (6CI,7CI,8CI) 4,5,6,7-Tetrabromobenzofuran-1,3-dione 4,5,6,7-Tetrabromoisobenzofuran-1,3-dione Bromphthal FG 4000 FireMaster PHT 4 NSC 4874 PHT 4 Saytex RB 49 Tetrabromophthalic acid anhydride Tetrabromophthalic anhydride |
R | 463.7 | 3.7 | 2450 | na | 1.27E-09 |
| 87-83-2 |
PBT PeBT |
PBT |

|
Pentabromotoluene 1.2.3.4.5-Pentabromo-6-methylbenzene 2.3.4.5.6-Pentabromomethylbenzene 2,3,4,5,6-Pentabromotoluene Flammex 5BT PBT Pentabromomethylbenzene |
A | 486.62 | 6.25 | 60,200 | na | 6.00E-04 |
| 608-71-9 |
PBP PeBPh |
PBP PBPh |

|
Pentabromophenol Phenol, pentabromo- (6CI,7CI,8CI,9CI) 2,3,4,5,6-Pentabromophenol Bromophenasic acid Flammex 5BP NSC 5717 Perbromophenol |
A/R | 488.59 | 5.22 | pH-dep. | 4.43±0.33 | 2.55E-03 |
| 85-22-3 |
PBEB PeBEtBz |
PeBrEtBz PBEB |

|
Pentabromoethylbenzene 2,3,4,5,6-Pentabromoethylbenzene EB 80 |
A | 500.65 | 6.76 | 1.14E+05 | na | 1.56E-04 |
| 58495-09-3 |
PBBC PeBBnC |

|
Pentabromobenzyl chloride 2,3,4,5,6-Pentabromobenzyl chloride |
R | 521.06 | 5.95 | 41,300 | na | 8.64E-06 | |
| 3555-11-1 |
PBP-AE PeBPh-AE |
PBPAE |

|
Pentabromophenol allyl ether (Allyloxy)pentabromobenzene Allyl pentabromophenyl ether Flammex 5AE Pentabromophenyl allyl ether |
A/R | 528.66 | 6.22 | 57,900 | na | 9.21E-05 |
| 35109-60-5 |
TBP-DBPE TrBPh-DiBPrE |
DPTE |

|
2,4,6-Tribromophenyl 2,3-dibromopropyl ether 2,3-Dibromopropyl 2,4,6-tribromophenyl ether Bromkal 73-5PE 1,3,5-tribromo-2-(2,3-dibromopropoxy)benzen |
A | 530.67 | 5.82 | 35,000 | na | 1.26E-05 |
| 183658-27-7 |
EH-TBB EtH-TeBBzo |
EHTeBB EHTBB TBB |

|
2-Ethylhexyl 2,3,4,5-tetrabromobenzoate | A | 549.92 | 7.73 | 3.82E+05 | na | 3.71E-07 |
| 87-82-1 |
HBB HxBBzb |
HBB HxBrBz |

|
Hexabromobenzene 1,2,3,4,5,6-Hexabromobenzene AFR 1001 FR-B HBB HBB (flame retardant) HBB-S NSC 113975 Perbromobenzene Plasafety HBB |
A | 551.49 | 6.11 | 50,300 | na | 1.14E-04 |
| 59447-55-1 |
PBB-Acr PeBBn-Acr |
PeBrAcr |

|
Pentabromobenzyl acrylate 2,3,4,5,6-Pentabromobenzyl acrylate Actimer FR 1025M FR 1025M |
R | 556.67 | 5.6 | 26,500 | na | 3.64E-07 |
| 38521-51-6 |
PBBB PeBBnB |
PBBB |

|
Pentabromobenzyl bromide 2,3,4,5,6-Pentabromobenzyl bromide |
R | 565.51 | 6.22 | 57,400 | na | 4.25E-06 |
| 20566-35-2 |
HEEHP-TEBP OHEtOEt-OHPr- TeBPht |
TeBrPht |

|
2-(2-Hydroxyethoxy)ethyl 2-hydroxypropyl 3,4,5,6-tetrabromophthalate Phthalic acid, tetrabromo-, 2-(2-hydroxyethoxy)ethyl 2-hydroxypropyl ester PHT 4-Diol Saytex RB 79 |
A | 627.9 | 1.04 | 87.5 | na | 4.79E-13 |
| 26040-51-7 |
BEH-TEBP bEtH-TeBPht |
TeBrDEHP TBPH BEHTBP |

|
Bis(2-ethylhexyl) tetrabromophthalate 1,2-Benzenedicarboxylic acid, 3,4,5,6-tetrabromo-, bis(2-ethylhexyl) ester (9CI) Phthalic acid, tetrabromo-, bis(2-ethylhexyl) ester (8CI) DP 45 Di(2-ethylhexyl) tetrabromophthalate Pyronil 45 Uniplex FRP 45 |
A | 706.14 | 9.34 | 2.88E+ 06 | na | 1.55E-11 |
| 168434-45-5 |
TBPD-TBP TeBPeDe-TrBPh |
TBPTP |

|
3-(Tetrabromopentadecyl)-2,4,6-tribromophenol | A/R | 856.78 | na | na | na | na |
| 79-94-7 | TBBPA b | TBBPA TBBP-A |

|
Tetrabromobisphenol A 2,2-Bis(3,5-dibromo-4-hydroxyphenyl)propane 2,2-Bis(4-hydroxy-3,5-dibromophenyl)propane 2,2′,6,6′-Tetrabromobisphenol A 3,3′,5,5′-Tetrabromobisphenol A 3,5,3′,5′-Tetrabromobisphenol A 4,4′-(1-Methylethylidene)bis[2,6-dibromophenol] 4,4′-Isopropylidenebis[2,6-dibromophenol] BA 59 BA 59BP BA 59P Bromdian CP 2000 FG 2000 FR 1524 Fire Guard 2000 Firemaster BP 4A Flame Cut 120G Flame Cut 120R GLCBA 59P NSC 59775 PB 100 RB 100 Saytex CP 2000 Saytex RB 100 Saytex RB 100PC T 0032 TBBPA Tetrabromodian Tetrabromodiphenylolpropane |
A/R | 543.87 | 9.69 | 4.47E + 06 | 7.5/8.50 ± 0.10 | 1.88E-05 |
| 39635-79-5 | TBBPS b | TBBPS TBBP-S |

|
Tetrabromobisphenol S 3,3′,5,5′-Tetrabromobisphenol S 3,5,3′,5′-Tetrabromobisphenol S 4,4′-Dihydroxy-3,5,3′,5′-tetrabromodiphenyl sulfone 4,4′-Sulfonylbis(2,6-dibromophenol) Bis(3,5-dibromo-4-hydroxyphenyl) sulfone Bis(4-hydroxy-3,5-dibromophenyl) sulfone EB 400S FG 400S Flame Cut 160R NFPP |
A/R | 565.85 | 5.81 | pH-dep, | 3.53 | 4.03E-10 |
| 37853-61-5 |
TBBPA-BME TBBPA-bMeEc |
TBBPA ME |

|
Tetrabromobisphenol A bismethyl ether Tetrabromobisphenol A dimethyl ether Tetrabromobisphenol A methyl ether |
A | 571.92 | 10.35 | 1.00E + 07 | na | 2.25E-06 |
| 70156-79-5 |
TBBPS-BME TBBPS-bMeEc |

|
Tetrabromobisphenol S bismethyl ether (3,5-Dibromo-4-methoxyphenyl) sulfone Tetrabromobisphenol S dimethyl ether Tetrabromobisphenol S methyl ether |
A | 593.91 | 6.05 | 46,500 | na | 3.43E-11 | |
| 33798-02-6 |
TBBPA-BOAc TBBPA-bOAcc |

|
3,3′,5,5′-Tetrabromobisphenol A bisacetate Phenol, 4,4′-(1-methylethylidene)bis[2,6- dibromo-, diacetate (9CI) Phenol, 4,4′-isopropylidenebis[2,6-dibromo-, diacetate (8CI) 2,2-Bis(4-acetoxy-3,5-dibromophenyl)propane 3,3′,5,5′-Tetrabromobisphenol A diacetate |
A | 627.94 | 9.45 | 3.28E+06 | na | 3.28E-09 | |
| 4162-45-2 |
TBBPA-BHEE TBBPA-bOHEtEc |
TBBPA-BHEE TBBPA OHEE |

|
Tetrabromobisphenol A bis(2-hydroxyethyl) ether Ethanol, 2,2′-[isopropylidenebis[(2,6-dibromo- pphenylene)oxy]]di- 2,2-Bis[3,5-dibromo-4-(2-hydroxyethoxy) phenyl]propane 2,2-Bis[3,5-dibromo-4-(p-hydroxyethoxy) phenyl]propane 2,2-Bis[4-(2-hydroxyethoxy)-3,5- dibromophenyl]propane 2,2′-Isopropylidenebis[(2,6-dibromo- pphenyleneoxy)diethanol] 4,4′-Isopropylidenebis[2-(2,6- dibromophenoxy)ethanol] AFR 1011 BA 50 BA 50P FG 3600 Fire Guard 3600 BA-EO 20 T |
A/R | 631.98 | 8.51 | 1.01E+06 | 13.76 | 2.89E-12 |
| 25327-89-3 |
TBBPA-BAE TBBPA-bAEc |
TBBPA-DAE TBBPA-AE |

|
Tetrabromobisphenol A bis(allyl) ether Benzene, 1,1′-(1-methylethylidene)bis [3,5-dibromo-4-(2-propenyloxy)- (9CI) Propane, 2,2-bis[4-(allyloxy)-3,5-dibromophenyl]-(8CI) 1,1′-Isopropylidenebis[4-(allyloxy)-3,5-dibromobenzene] 2,2-Bis(3,5-dibromo-4-allyloxyphenyl)propane 2,2-Bis(4-allyloxy-3,5-dibromophenyl)propane BE 51 FG 3200 Fire Guard 3200 Flame Cut 122K Pyroguard SR 319 SR 319 TBBPA-DE Tetrabromobisphenol A allyl ether Tetrabromobisphenol A diallyl ether |
A/R | 642 | 11.42 | 1.00E + 07 | na | 1.83E-08 |
| 55205-38-4 |
TBBPA-BA TBBPA-bAcrc |

|
Tetrabromobisphenol A bisacrylate 2-Propenoic acid, (1-methylethylidene) bis(2,6-dibromo-4,1-phenylene) ester (9CI) 2,2′,6,6′-Tetrabromobisphenol A diacrylate 4,4′-Isopropylidenebis(2,6-dibromophenyl aciylate) SR 640 Tetrabromobisphenol A diacrylate |
R | 651.97 | 9.37 | 2.99E + 06 | na | 3.84E-11 | |
| 3072-84-2 |
TBBPA-BGE TBBPA-bGEc |
TBBPA-DGE TBBPA GE |
Oxirane, 2,2′-[(1-methylethylidene) bis[(2,6-dibromo-4,1- phenylene)oxymethylene]]bis- 
|
Tetrabromobisphenol A bis(glycidyl) ether Propane, 2,2-bis[3,5-dibromo-4- (2,3-epoxypropoxy)phenyl]- (7CI,8CI) 2,2-Bis(4-glycidyloxy-3,5-dibromophenyl)propane 2,2′,6,6′-Tetrabromobisphenol A diglycidyl ether Glycidyl tetrabromodian ether Tetrabromobisphenol A diglycidyl ether |
R | 656 | 8.87 | 1.60E + 06 | na | 1.64E-10 |
| 37419-42-4 |
TBBPA-BP TBBPA-bPrtc |

|
Tebrabromobisphenol A bispropanoate propane-2,2-diylbis(2,6-dibromo-4,1-phenylene) dipropionate |
A | 656 | 10.47 | 1.00E + 07 | na | 4.17E-10 | |
| 37853-59-1 |
BTBPE bTBPhOEtc |
BTBPE TBEHxBrPoxE |

|
1,2-Bis(2,4,6-tribromophenoxy)ethane BTBPE FF 680 FI 680 FM 680 FireMaster 680 FireMaster FF 680 |
A | 687.64 | 8.31 | 7.92E + 05 | na | na |
| 66710-97-2 |
TBBPA-BHEEBA TBBPA-bOHEtEbAcrc |

|
Tetrabromobisphenol A bis(2-hydroxyethyl)ether bisacrylate 2-Propenoic acid, (1-methylethylidene)bis[(2,6- dibromo-4,1-phenylene)oxy-2,1- ethanediyl] ester (9CI) BABA 50 |
R | 740.07 | 10.76 | 1.00E +07 | na | 1.96E-14 | |
| 1084889-51-9 1025956-65-3 893843-07-7 |
OBTMPI OBTrMePhIna |
OBIND OctaInd Br-Indane |

|
Octabromotrimethylphenyl indane OctaInd 4,5,6,7-tetrabromo-1,1,3-trimethyl-3-(2,3,4,5- tetrabromophenyl)-2,3-dihydro-1H-indene |
A | 867.52 | 15.11 | 1.00E + 07 | na | 1.75E-12 |
| 21850-44-2 |
TBBPA-BDBPE TBBPA-bDiBPrEc |
TBBPA-DBPE TBBPA-bis |

|
Tetrabromobisphenol A bis(2,3- dibromopropyl) ether Propane, 2,2-bis[3,5-dibromo-4-(2,3- dibromopropoxy)phenyl]- (8CI) 1,1′-Isopropylidenebis[3,5-dibromo-4- (2,3-dibromopropoxy)benzene] 2,2-Bis[3,5-dibromo-4-(2,3- dibromopropoxy)phenyl]propane 2,2-Bis[4-(2,3-dibromopropoxy)-3, 5-dibromophenyl]propane 2,2-Bis[4-(2,3-dibromopropyloxy)-3, 5-dibromophenyl]propane 2,2-Bis[[3,5-dibromo-4-(2,3- dibromopropyloxy)]phenyl]propane 3,3′,5,5′-Tetrabromobisphenol A bis(2,3- dibromopropyl) ether 4,4′-Isopropylidenebis[2,6-dibromo-1-(2,3- dibromopropoxy)benzene] Bis(2,3-dibromopropoxy)tetrabromobisphenol A Bromkal 66-8 D 5532 FG 3100 FR 720 Fire Guard 3100 Flame Cut 121K Flame Cut 121R GX 5532y HP 800A PE 68 PE 68 (fireproofing agent) Pyroguard SR 720 SR 720 Saytex HP 800A Saytex HP 800AG TBBPA-DBPE Tetrabromobisphenol A 2,3- dibromopropyl ether |
A | 943.61 | 12.99 | 1.00E +07 | na | 2.85E-15 |
| 32588-76-4 |
EBTEBPI N,N’-EtbTeBPhtIm |
BrPhtimi |

|
N,N’-Ethylenebis(tetrabromophthalimide) Phthalimide, N,N’-ethylenebis[tetrabromo- (8CI); 1,2- Bis(tetrabromophthalimido)ethane BT 93 BT 93W BT 93WFG Citex BT 93 Saytex BT 93 Saytex BT 93W 2,2′-(ethane-1,2-diyl)bis(4,5,6,7-tetrabromoisoindoline-1,3-dione) |
A | 951.47 | 6.63 | 96,500 | na | 1.97E-25 |
| 42757-55-1 |
TBBPS-BDBPE TBBPS-bDiBPrEc |

|
Tetrabromobisphenol S bis(2,3-dibromopropyl ether) 4,4′-Bis(2,3-dibromopropoxy)-3,3′,5,5′- tetrabromodiphenyl sulfone Bis[3,5-dibromo-4-(2,3- dibromopropoxy)phenyl] sulfone Flame Cut 161R Nonnen 52 Nonnen PR 2 PR 2 |
A | 965.6 | 8.68 | 1.26E+06 | na | 1.65E-21 | |
| 84852-53-9 |
DBDPE BDPE-209 DBDiPhEtb |
DBDPE DBDE EBPE DeBrPylE |

|
Decabromodiphenyl ethane | A | 971.22 | 11.1 | 1.00E + 07 | na | na |
| 497107-13-8 |
DBDBE BDBE-209 DBDiBnE |
DBDBE |

|
Decabromodibenzyl ether Bis(2,3,4,5,6-pentabromobenzyl) ether |
A | 987.22 | 10.34 | 9.99E + 06 | na | 2.31E-16 |
| PBBs b |

|
Polybrominated biphenyls | A | |||||||
| PBDEs b |

|
Polybrominated diphenyl ethers Polybrominated phenoxy benzenes Polybrominated diphenyl oxides |
A | |||||||
| 58965-66-5 |
4′-PeBPOBDE208 TeDB-DiPhOBzd |
DPeTeBrBz |

|
Tetradecabromo-1,4-diphenoxybenzene Bis(pentabromophenoxy)benzene 1,4-Bis(pentabromophenoxy)tetrabromobenzene BT 120 Saytex 120 Pentabromophenoxy-nonabromodiphenyl ether |
A | 1366.85 | 12.67 | 1.00E+07 | 9.17E-17 | |
| 34571-16-9 |
HCTBPH HxCTeBPh-bcHen |
Dec 604 |

|
1,2,3,4,7,7-hexachloro-5-(2,3,4,5- tetrabromophenyl)-Bicyclo[2.2.1]hept-2-ene 2-Norbornene, 1,2,3,4,7,7-hexachloro- 5-(tetrabromophenyl)- (8CI) 5-(Tetrabromophenyl)-1,2,3,4,7,7- hexachloro-2-norbornene Dechlorane 604 Component A Hexachlorocyclopentadiene- tetrabromostyrene adduct Dechlorane 604 |
A | 692.5 | 10.24 | 8.86E+06 | na | 1.61E-08 |
| 3322-93-8 |
DBE-DBCH DiBEt-DiBcH |
TBEC TBECH BrCyHx |
|
4-(1,2-Dibromoethyl)-1,2-dibromocyclohexane 1-(1,2-Dibromoethyl)-3,4-dibromocyclohexane 1,2-Dibromo-4-(1,2-dibromoethyl)cyclohexane Saytex BCL 462 Citex BCL 462 |
A | 427.8 | 4.82 | 10,000 | na | 2.97E-03 |
| 3194-57-8 |
TBCO α-/β-TeBcO |
TBCO |

|
1,2,5,6-Tetrabromocyclooctane NSC 167079 |
A | 427.8 | 5.28 | 17,800 | na | 4.79E-03 |
| 51936-55-1 |
DBHCTD DiBHxC-TrcTrDen |
HCDBCO |

|
5,6-Dibromo-1,10,11,12,13,13-hexachloro- 11-tricyclo[8.2.1.02,9]tridecene 7,8-dibromo-1,2,3,4,11,11-hexachloro- 1,4,4a,5,6,7,8,9,10,10a-decahydro- 1,4-methanobenzo[8]annulene* Saytex BC 26 Citex BC 26 * Name as suggested by ChemDraw |
A | 540.76 | 7.62 | 3.32E+05 | na | 8.27E-07 |
| 25495-98-1 |
HBCYD HxBcDea |
HBCD |

|
Hexabromocyclodecane | A | 613.64 | na | na | na | na |
| 3194-55-6 |
HBCDD (HBCD) HxBcDDa,b |
HBCDD HBCD |
Cyclododecane, 1,2,5,6,9,10-hexabromo-
|
1,2,5,6,9,10-Hexabromocyclododecane Bromkal 73-6D FR 1206 FR 1206HT Pyroguard SR 104 SR 104 YM 88A |
A | 641.7 | 7.92 | 4.86E+05 | na | 1.04E-07 |
| 57829-89-7 |
DBP-TAZTO DiBPr-DiA-Tazto |

|
1-(2,3-Dibromopropyl)-3,5-diallyl-1,3,5- Triazine-2,4,6(1H,3H,5H)-trione 1-(2,3-Dibromopropyl)-3,5-di-2-propenyl-1,3,5- Triazine-2,4,6(1H,3H,5H)-trione |
A/R | 409.07 | 2.66 | 667 | na | 2.16E-06 | |
| 75795-16-3 |
BDBP-TAZTO bDiBPr-A-Tazto |

|
1,3-Bis(2,3-dibromopropyl)-5-allyl-1,3,5-Triazine- 2,4,6(1H,3H,5H)-trione 1,3-Bis(2,3-dibromopropyl)-5-(2-propen-1-yl)- 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione 1-Allyl-3,5-bis(2,3-dibromopropyl)- 1,3,5-triazinane- 2,4,6-trione |
A/R | 568.88 | 3.55 | 2040 | na | 1.19E-09 | |
| 52434-90-9 |
TDBP-TAZTO tDiBPr-Tazto |
TBC BrTriaz |

|
1,3,5-Tris(2,3-dibromopropyl)-1,3,5-triazine- 2.4.6- trione 1,3,5-Tris(2,3-dibromopropyl) isocyanurate 1,3,5-Tris(2,3-dibromopropyl)-2,4,6- trioxohexahydrotriazine AFR 1002 FC 140R FCP 660CN FR 930 Fire Cut P 660 Fire Cut P 660CN TAIC 6B TBC TBC (fireproofing agent) Tris(2,3-dibromopropyl) isocyanurate Tris(2,3-dibromopropyl) isocyanurate |
A | 728.67 | 4.45 | 6260 | na | 4.09E-13 |
| 25713-60-4 |
TTBP-TAZ tTrBPhO-Taz |
BrPhTriaz |

|
Tris(2,4,6-tribromophenoxy)-s-triazine s-Triazine, 2,4,6-tris(2,4,6-tribromophenoxy)- (8CI) 2,4,6-Tris(2,4,6-tribromophenoxy)-1,3,5-triazine FR 245 FR 368 GX 6145 Pyroguard SR 245 SR 245 |
A | 1067.43 | 12.97 | 1.00E+07 | na | 2.69E-23 |
| 19186-97-1 |
TTBNPP tBbBMePrP |
TrisPhos TTBNP |

|
Tris(tribromoneopentyl) phosphate Tris[3-bromo-2,2-bis(bromomethyl)propyl] phosphate 1-Propanol, 3-bromo-2,2-bis(bromomethyl)-, phosphate (3:1) CR 900 FR 370 FR 372 Flame Cut 175 Flame Cut 175R Kronitex PB 370 PB 370 Reoflam FR 370 TPB 3070 Tris[2,2-bis(bromomethyl)-3- bromopropyl] phosphate |
A | 1018.46 | 7.55 | 3.04E+05 | na | 1.41E-17 |
| 126-72-7 |
TDBPP tDBPrP |
TDBPP |

|
Tris(2,3-dibromopropyl) phosphate Fyrol HB 32 |
A | 697.61 | 3.71 | 2485 | na | 3.17E-09 |
| 3296-90-0 |
DBNPG bBMe-PrDiOH |
DBPT |
|
Dibromoneopentyl glycol 2.2-Bis(bromomethyl)-1,3-propanediol 1.3-Dibromo-2,2-bis(hydroxymethyl)propane 1,3-Dibromo-2,2-dihydroxymethylpropane 1,3-Dibromo-2,2-dimethylolpropane 2,2-Dibromomethyl-1,3-propanediol FR 1138 FR 522 NSC 9001 Pentaerythritol dibromide Pentaerythritol dibromohydrin |
R | 261.94 | 0.41 | 39.9 | 13.57 | 6.88E-05 |
| 522-92-5 |
TBNPA tBMe-EtOH |
TBPT |
|
2,2,2-Tris(bromomethyl)ethanol Tribromoneopentyl alcohol 2,2-Bis(bromomethyl)-3-bromo-1-propanol 3-Bromo-2,2-bis(bromomethyl)-1-propanol 3-Bromo-2,2-bis(bromomethyl)propanol 3-Bromo-2,2-bis(bromomethyl)propyl alcohol FR 1360 FR 513 NSC 20521 Pentaerythritol tribromide Pentaerythritol tribromohydrin |
R | 324.84 | 2.06 | 315 | 13.73 | 1.60E-03 |
It is possible to add the positions for the bromine substituents in front of the abbreviation.
Well established abbreviation. No change is proposed.
TBBPA or TBBPS derivatives are constructed as a mix of the traditional abbreviations and the novel abbreviations are added for the functionalisation of the hydroxyl group.
PBPhO-PBDE stands for polybromophenoxy-PBDE, in analogy with e.g. MeO-PBDE.
Table 4.
Practical abbreviation (PRAB, in bold) for phosphorus containing flame retardants, together with structured abbreviations (STAB; plain text) are presented, The table also includes some basic physicochemical constants calculated using ACD/Labs Software V11.02. The STABs are constructed as described under “Methodology”, incl. Table 1.
| CAS number | PRABs STABs |
Previously used abbreviations |
CA name | Common and trade names | MW | Log Kow | Koc | Vapor pressure (Pa) |
|---|---|---|---|---|---|---|---|---|
| 512-56-1 |
TMP tMeP |
TMP TMPA TMPO |

|
Tris(methyl) phosphate Trimethyl phosphate CCRIS 610 NSC 58985 Phosphoric acid, trimethyl ester NCI-C03781 Trimethoxyphosphineoxide Methyl phosphate |
140.08 | −0.65 | 3.2–12 | 1.13E+02 |
| 78-40-0 |
TEP tEtP |
TEP |

|
Tris(ethyl) phosphate Triethyl phosphate Ethyl phosphate Aurora KA-1638 Fyrol TEP Phosphoric acid, triethyl ester |
182.16 | 0.80 | 36 | 5.24E+01 |
| 513-08-6 |
TPP tPrP |
TnPP TPrP |

|
Tris(propyl) phosphate Tri-n-propyl phosphate TPP Propyl phosphate Tripropyl phosphate Phosphoric acid tri-n-propyl ester Tris(n-propyl) phosphate |
224.24 | 1.87 | 676 | 5.77E-01 |
| 126-71-6 |
TIBP tiBuP |
TiBP TIP |

|
Tris(isobutyl) phosphate Phosphoric acid, triisobutyl ester Tris(2-methylpropyl) phosphate Antifoam TIP Daiguard 400 NSC 62222 Reomol TIBP |
266.32 | 3.60 | 977 | 1.71E+00 |
| 126-73-8 |
TNBP tBuP |
TnBP TBP TB |

|
Tris(butyl) phosphate Tri-n-butyl phosphate MCS2495 Butyl phosphate Celluphos 4 Disflamoll TB Kronitex TBP Phos-Ad 100 Antifoam T Tributylphosphate Phosphoric acid, tri-n-butyl ester |
266.32 | 4.00 | 3600 | 1.51E-01 |
| 78-51-3 |
TBOEP tBuOEtP |
TBEP TBXP |

|
Tris(2-butoxyethyl) phosphate Ethanol,2-butoxy-, phosphate (3:1) Phosphoric acid, tris(2-butoxyethyl)ester 2-Butoxyethanol phosphate Amgard TBEP Hostaphat B310 Ethanol, 2-butoxy-,1,1′,1′′-phosphate Phosflex T-BEP Tris(2-butoxyethyl) phosphate Tris(2-n-butoxyethyl) phosphate Tris(butoxyethyl) phosphate |
398.48 | 3.75 | 1020 | 3.33E-06 |
| 78-42-2 |
TEHP tEtHP |
TEHP TOF TOP |

|
Tris(2-ethylhexyl) phosphate 2-Ethylhexanol,phosphate (3:1) DisflamollTOF Trioctyl phosphate Tris(2-ethylhexyl) phosphate Durad TOP Flexol TOF NSC 407921 Reomol TOF |
434.64 | 9.49 | 617,000 | 1.10E-05 |
| 115-96-8 |
TCEP tCEtP |
TCEP |

|
Tris(chloroethyl) phosphate 3CF Amgard TCEP CEF CLP Celluflex CEF Disflamoll TCA Fyrol CEF Fyrol CF Genomoll P NSC 3213 Niax 3CF Niax Flame Retardant 3CF Tris(2-chloroethyl) phosphate Tris(chloroethyl) phosphate Tris(ß-chloroethyl) phosphate Tris(2-chloroethyl) orthophosphate Tris(2-chloroethyl) phosphate Tris(ß-chloroethyl) phosphate |
285.49 | 1.47 | 150 | 1.44E-02 |
| 13674-84-5 |
TCIPP tCiPP |
TCPP TCiPP |

|
Tris(2-chloroisopropyl) phosphate 2-Propanol, 1-chloro-, phosphate (3:1) Fyrol PCF Antiblaze 80 1-Chloro-2-Propanol Phosphate (3:1) Amgard TMCP Hostaflam OP 820 Phosphoric acid, tris(2-chloro-1-methylethyl) ester Tri-(2-chloroisopropyl) phosphate Tris(1-chloro-2-propyl) phosphate Tris(2-chloro-1-methylethyl) phosphate Tris(β-chloropropyl) phosphate Tris(chloroisopropyl) phosphate |
327.56 | 2.59 | 275 | 2.69E-03 |
| 78-43-3 |
TDCPP tDiCPrP |
TDCPP | 1-Propanol, 2,3-dichloro-, 1,1′,1′′-phosphate
|
Tris(2,3-dichloropropyl) phosphate 1-Propanol, 2,3-dichloro-, phosphate (3:1) (8CI,9CI) Celluflex FR 2 Fyrol 32B Tris(2,3-dichloropropyl) phosphate Tris(2,3-dichloro-n-propyl) phosphate |
430.9 | 2.98 | 998 | 8.67E-07 |
| 13674-87-8 |
TDCIPP tDiCiPP |
TDCPP TDCiPP |

|
Tris(1,3-dichloroisopropyl) phosphate 3PC-R Antiblaze 195 CRP CRP (fireproofing agent) FR 10 FR 10 (phosphate) Fyrol FR 2 PF 38 PF 38/3 TDCPP Tris(1,3-dichloro-2-propyl) phosphate Tris(1-chloromethyl-2-chloroethyl)phosphate Tris[2-chloro-1-(chloromethyl)ethyl] phosphate |
430.9 | 3.27 | 1440 | 5.43E-06 |
| 38051-10-4 | BCMP-BCEPbCMePrDiOHbbCEtP |

|
2,2-Bis(chloromethyl)-1,3-propanediol bis[bis(2-chloroethyl) phosphate] Phosphoric acid, 2,2-bis(chloromethyl)-1, 3-propanediyl tetrakis(2-chloroethyl) ester (9CI) Phosphoric acid, bis(2-chloroethyl) ester, diester with 2,2-bis(chloromethyl)-1,3-propanediol (7CI) 2,2-Bis(chloromethyl)-1,3-propanediol 2-chloroethanol phosphate (1:4:2) 2,2-Bis(chloromethyl)-1,3-propanediyl bis[bis(2-chloroethyl) phosphate] Amgard V 6 Antiblaze 100 Antiblaze AB 100 Antiblaze V 6 Phosgard 2XC20 |
582.99 | 2.52 | 557 | 1.61E-12 | |
| 1047637-37-5 |
BCMP-BCMEP bCMePrDiOHbb CMeEtP |
U-OPFR | Phosphoric acid, P,P′-[2,2-bis(chloromethyl)-1, 3-propanediyl] P,P,P′,P′-tetrakis(2-chloro- 1-methylethyl) ester 
|
2,2-Bis(chloromethyl)-1,3-propanediol bis[bis(2-chloro1-methylethyl) phosphate] 2,2-Bis(chloromethyl)propane-1,3-diyl tetrakis(1-chloropropan-2-yl) bis(phosphate) |
639.1 | 3.93 | 3280 | 1.96E-13 |
| 126-72-7 | TDBPP |
TDBPP |

|
Tris(2,3-dibromopropyl) phosphate 1-Propanol, 2,3-dibromo-, phosphate (3:1) (6CI,8CI,9CI) Phosphoric acid, tris(2,3-dibromopropyl) ester (6CI) 3PBR Anfram 3PB Apex 462-5 Bromkal P 67-6HP ES 685 FireMaster LV-T 23P FireMaster T 23 FireMaster T 23P Flammex AP Flammex LV-T 23P Flammex T 23P Fyrol HB 32 NSC 3240 Phoscon FR 150 Phoscon PE 60 Phoscon UF-S T 23P TDBPP Tris Tris (flame retardant) Zetofex ZN |
697.61 | 3.71 | 2485 | 3.17E-09 |
| 19186-97-1 |
TTBNPP tBbBMe-PrP |
CR 900 FR 370 |

|
Tris(tribromoneopentyl) phosphate Tris[3-bromo-2,2-bis(bromomethyl)propyl] phosphate 1-Propanol, 3-bromo-2,2-bis(bromomethyl)-, phosphate (3:1) CR 900 FR 370 FR 372 Flame Cut 175 Flame Cut 175R Kronitex PB 370 PB 370 Reoflam FR 370 TPB 3070 Tris[2,2-bis(bromomethyl)-3-bromopropyl] phosphate |
1018.46 | 7.55 | 3.04E+05 | 1.41E-17 |
| 46355-07-1 | IPPP iPrPhP |
Phosphoric acid, mono(1-methylethyl) monophenyl ester 
|
Isopropyl phenyl phosphate | 216.17 | 1.71 | na | 5.49E-02 | |
| 35948-25-5 |
DOPO DiBzOPO |
DOPO |

|
3,4:5,6-Dibenzo-2H-1,2-oxaphosphorin-2-oxide 6-Hydroxy-6H-dibenz[c,e][1,2]oxaphosphorin 9,10-Dihydro-9-oxa-10-phosphaphenanthren-10-oxide 9,10-Dihydro-9-oxa-10-phosphaphenanthrene 10-oxide 9,10-Dihydro-9-oxa-10-phosphorylphenanthrene-10-oxide DOPO HCA HCA (heat stabilizer) Hiretar 101 Sanko HCA Ukanol DOPO Ukanol GKF |
216.17 | na | na | 4.15E-04 |
| 115-86-6 |
TPHP tPhP |
TPP TPhP |

|
Tris(phenyl) phosphate Triphenyl phosphate Phosphoric acid, triphenyl ester Altal Dymel Phosflex TPP Kronitex TPP Celluflex TPP Disflamoll TP |
326.29 | 4.59 | 2630 | 8.37E-04 |
| 1330-78-5 |
TMPP tMePhP |
TCP TTP TBT |

|
Tris(methylphenyl) phosphate Tricresyl phosphate (mixture of ortho, meta, para) TBT Cresyl phosphate Tritolyl phosphate Phosphoric acid, tricresyl ester Phosphoric acid, tritolyl ester Celluflex179c Disflamolltkp Durad Flexolplasticizer TCP Fyrquel150 Imols140 Kronitex Lindol Nci-c61041 Phosflex179a |
368.37 | 5.11 | 4680 | 8.00E-05 |
| 2502-15-0 |
TIPPP tiPrPhP |

|
Tris(4-isopropylphenyl) phosphate Phenol, p-isopropyl-, phosphate (3:1) (8CI) p-Cumenyl phosphate ((C9H11O)3PO) (7CI) Tris(p-isopropylphenyl) phosphate |
452.52 | 6.75 | 1.12E+05 | 3.89E-07 | |
| 57583-54-7 |
PBDPP RebDiPhP |

|
Resorcinol bis(diphenyl phosphate) Phosphoric acid, 1,3-phenylene tetraphenyl ester (9CI) 1,3-Phenylene bis(diphenyl phosphate) ADK Stab PFP ADK Stab PFR BPHPPO CR 733S Fyrolflex RDP Mark PFK Nonnen R 0111-10 PFR Reofos RDP Resorcinol tetraphenyl diphosphate Tetraphenyl m-phenylene bisphosphate Tetraphenyl m-phenylene diphosphate Tetraphenyl resorcinol bis(diphenyl phosphate) Tetraphenyl resorcinol diphosphate WSFR-RDP m-Phenylene bis(diphenyl phosphate) |
574.46 | 7.08 | 1.69E+05 | 5.01E-11 | |
| 139189-30-3 |
PBDMPP RebDiDiMePhP |

|
Resorcinol bis[di(2,6-dimethylphenyl) phosphate] Phosphoric acid, 1,3-phenylene tetrakis (2,6-dimethylphenyl) ester (9CI) 1,3-Phenylenebis(2,6-dimethylphenyl phosphate) ADK Stab FP 500 FP 500 PX 200 PX 200 (phosphate) Resorcinol bis(di-2,6-xylyl phosphate) Resorcinol bis[bis(2,6-dimethylphenyl) phosphate] Tetrakis(2,6-dimethylphenyl) m-phenylene bisphosphate Tetrakis(2,6-dimethylphenyl) m-phenylene diphosphate Tetrakis(2,6-xylyl) m-phenylene diphosphate |
686.67 | 10.28 | 9.34E+06 | 4.44E-13 | |
| 5945-33-5 |
BPA-BDPP BPAbDiPhP |

|
Bisphenol A bis(diphenyl phosphate) Phosphoric acid, (1-methylethylidene)di-4, 1-phenylene tetraphenyl ester (9CI) Phosphoric acid, diphenyl ester, diester with 4,4′-isopropylidenediphenol (7CI) Phosphoric acid, isopropylidenedi-p-phenylene tetraphenyl ester (8CI) 2,2-Bis[4-[bis(phenoxy)phosphoryloxy]phenyl]propane 4,4′-(Isopropylidenediphenyl) bis(diphenyl phosphate) ADK Stab FP 600 ADK Stab FP 700 BADP BDP BPA-DP Bisphenol A tetraphenyl diphosphate CG 963 CR 741S CR 742 E 890 FP 600 FP 700 FP 750 Fyrolflex BDP NcendX P 30 Ncendex P 30 Resorcinol bis(diphenyl phosphate)-Bisphenol A Bis(diphenyl phosphate) copolymer Tetraphenyl bisphenol A bisphosphate WSFR-BDP |
692.63 | 8.29 | 7.70E+05 | 1.97E-15 |
No inorganic FRs have been included in the present article since we feel that the chemical formula can be used for most of those chemicals.
2.1. Construction of STABs for BFRs, CFRs, and PFRs
Abbreviations should, as far as possible, be based on a “readable” common name of the chemical. This may lead to the use of an abbreviation, such as TBBPA originating from the common name tetrabromobisphenol A. The goal is to minimize use of non-interpretable names as a base of the abbreviation if it is possible to do so. However, some names and structures of the FRs are very complex and it is unavoidable that the STABs also become complex.
Functional groups, such as ether and ester groups, and glycidyl and allyl groups, should be handled the same way each time such a group appears in a compound. Alcohol functional groups are added as OH to the aliphatic chain name (e.g. MeOH for methanol, EtOH for ethanol, PrOH for propanol and PrDiOH for propanediol).
In cases where it is necessary to indicate the aliphatic chain or ring structure, this can be done by adding the lower case letters c for cyclo, bc for bicyclo; i for iso (c.f. Table 2). The default for an alkyl chain is “normal-” (n) and is omitted.
The “bis-” and “tris-” prefixes are written as “b” and “t”, respectively.
The numbers of a particular substituent are given by the letters: Di; Tr; Te; Pe; Hx; Hp; O; N; D; UD; DD; TrD; TeD; for the series of 2–14 substituents.
The aliphatic chains or rings and aromatic entities are presented in Table 1.
2.2. Construction of PRABs for BFRs, CFRs, and PFRs
Since the STABs tend to be quite complicated, in numerous cases, we are proposing combinations of, in general, three to eight capital letters for PRABs. The PRABs take into account previously used abbreviations and shortening of the STABs. In a few cases the suggested PRABs exceed eight letters, but this is in cases where no other possibility was obvious to us. The goal has been to present PRABs that are derived in a logical manner (based on the STABs) and are expected to be adopted by the scientific community.
3. Discussion
Among the FRs discussed in this article, we propose a hierarchy for clarification of the status of these chemicals in an environment and health perspective. First, it may be worth to stress that there is a difference in the definition of e.g. an “emerging chemical pollutant” and an “emerging issue”. Further, an “established pollutant” could of course be an “emerging issue”. Hence the following definitions are put forward for any FRs:
Established FRs (BFRs/CFRs/PFRs) are chemicals which are extensively documented regarding production and use as FRs, chemistry, fate, exposures, environment and health issues (i.e. (eco-)toxicity and/or human health effects).
Emerging FRs (BFRs/CFRs/PFRs) are chemicals which are documented regarding production and use as FRs that have been shown to occur/distribute to the environment and/or wildlife, humans or other biological matrices.
Novel FRs (BFRs/CFRs/PFRs) are chemicals which are documented as potential FRs that have been shown to be present in materials or products.
Potential FRs (BFRs/CFRs/PFRs) are chemicals reported to have applications as FRs (e.g. in patents).
The numbers of established, emerging, novel and/or potential BFRs, CFRs and PFRs identified and reported in this paper are 55, 18 and 23, respectively (Tables 2–4). These numbers do not include either congeners or enantiomers of a given FR. The DBP-TAZTO and its two congeners, BDBP-TAZTO and TDBP-TAZTO, are listed with their separate CAS numbers in Table 2, even though these homologues most likely occur together in the same technical BFR product. On the other hand, we list PBDEs as one group of BFRs (Table 2), chlorinated paraffins as three groups (SCCP; MCCP and LCCP), depending on alkane chain lengths even though they have separate CAS numbers (Table 3).
The use of a numbering system as proposed by Ballschmiter and Zell (1980) for the PCB congeners made a major impact on all subsequent discussions of this group of chemicals (Ballschmiter et al., 1992). Since PBBs and PBDEs are also dicyclic aromatic compounds, it has been possible to replicate the PCB numbering system for the PBBs and PBDEs. The same method for abbreviations is proposed herein for polybrominated diphenyl ethanes (PBDPE) and polybrominated dibenzyl ethanes (PBDBE), since these compounds are likewise, dicyclic aromatic chemicals.
The numbering system proposed by Ballschmiter et al., has also become valuable for referring to metabolites of PCBs, PBBs and PBDEs. The rules to apply are given in Textbox 1, referring to the work by Letcher et al. (2000). The same numbering system can be applied to the polybrominated phenoxy-PBDEs (PBPO-PBDE) (see Table 2).
The PCB-based numbering system cannot unfortunately be applied to any other of the BFRs, CFRs or PFRs. The proposed PRABs for the BFRs, CFRs and PFRs are given in bold in Tables 2, 3 and 4, respectively. The background for selection of the PRABs is given above. The structures of each of the BFR, CFR and PFR compounds are also shown within Tables 2–4, respectively, together with the chemical abstract name and their CAS number.
STABs of BFRs, CFRs and PFRs are also given in Tables 2–4 (under the practical abbreviations (plain text)). These abbreviations follow the criteria set up above, as far as possible. For most of the BFRs, CFRs and PFRs, this yields abbreviations that are easily interpretable in relation to the compound’s structure and at least one of its chemical names. The name used as a basis for the STABs is shown first in the column presenting “Common names/Trade names” in Tables 2–4. In cases where the abbreviation criteria have not been followed, this is commented on in footnotes (Table 2).
Several of the abbreviations are based on abbreviations which have already been in common use for a long time, described as established abbreviations. In such cases we are not proposing changes to the abbreviations already in use. This leads, for example, to the use of TBBPA as part of the abbreviated name of each of its derivatives, but the attached functional group is abbreviated following the guidelines presented herein. We suggest, however, that the common abbreviation HBCD be changed to HBCDD, to avoid future intermix with hexabromocyclodecane (c.f. Table 2). However, since HBCD is so commonly used for hexabromocyclododecane, we do foresee that this abbreviation may be used also in the future. Therefore, we introduce HBCYD as the PRAB for hexabromocyclodecane. In addition to the specific recommendations given above, we also propose “PentaBDE”, “OctaBDE” and “DecaBDE” when referring to the corresponding commercial products.
Chemicals belonging to the BFRs and CFRs are listed in Tables 2 and 3 respectively, presenting the proposed PRABs and STABs, other abbreviations that have been used previously, chemical abstract name, CAS number, and common names/commercial names. The type of FR is indicated as “R” for “Reactive BFR/CFR” and “A” for “Additive BFR/CFR”. In an additional few columns are some properties of the individual compounds given, as extracted from CA (Scifinder, 2012) under the CAS number given in the table. The BFRs presented in Table 2 are structured as follows, with increasing molar masses for each subgroup:
Aromatic BFRs One aromatic ring compounds Benzenes, including alkyl substituted benzenes Phenols (simple) and one ring phenols being derivatized Benzoic acid esters and phthalate esters Two ring aromatic systems Neutrals aromatics (PBB and PBDEs, polybrominated trimethylophenyl indanes, others) TBBPA TBBPS and derivatives Three ring aromatics
BFRs containing both aromatic and cycloaliphatic structures
Cycloaliphatic BFRs
Heterocyclic BFRs (triazine rings)
Brominated phosphate esters as BFRs
Aliphatic BFRs
The BFRs are characterized by moderate to very high log Kow, with very few exceptions. Four of the BFRs listed are phenolic chemicals, two are one-phenyl ring compounds and two are bisphenols, which leads to a pH-dependent water solubility for each of these chemicals.
CFRs are listed in Table 3. The table is organized in a similar manner as Table 2, starting with aromatic CFRs and ending with aliphatic CFRs. The CFRs are also characterized by intermediate to high log Kow constants.
PFRs are listed in Table 4. The PFRs are presented in two groups, those containing an aromatic part (substituent) and those with only aliphatic ester groups, potentially bearing halogen substituents. Some of the PFRs also contain chlorine substituents, which enhance their log Kow, and possibly their bioaccumulation potential (van der Veen and de Boer, 2012).
Finally, it is our hope that the proposed PRABs for BFRs, CFRs and PFRs, in this document, will result in a general acceptance and use among scientists and stakeholders in the field. If used as proposed, it will result in less confusion when BFRs, CFRs or PFRs are being reported, even though the abbreviations may, in a few cases, be perceived as somewhat complicated.
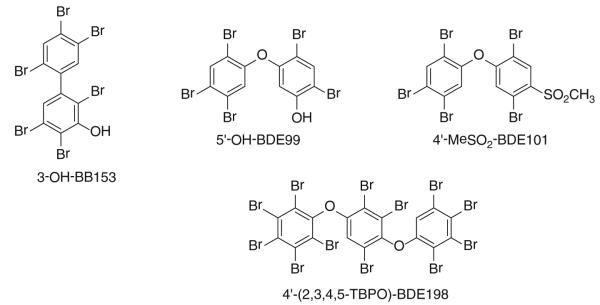
Determine the PBDE or PBB number of the OH-BDE, OH-BB or PhO-BDE overlooking any hetero substituent (−OH, −OR, −SH, −OR, −SR or PhO-group)
Based on the numbering of the PBDE or PBB congener, give the hetero substituent the number (with or without the prime sign due to the structure) in which the substituent is placed.
Examples of the numbering of PBDE and BB metabolites are given in Fig. 1, and likewise of a polybromophenoxy-PBDE (PBPO-PBDE) congener.
Fig. 1.
Examples of abbreviations for PBB and PBDE metabolites, as of PBPhO-BDEs. The appropriate abbreviations are given under each structure.
Acknowledgments
NVDE and AC acknowledge PhD and post-doctoral fellowships from the Flanders Research Foundation (FWO). AR acknowledges faculty funding from Stockholm University and Stockholm University’s Strategic Marine Environmental Research Funds through the Baltic Ecosystem Adaptive Management (BEAM). The opinions expressed here are those of the authors and do not necessarily reflect the views of the California Department of Toxic Substances Control, or of NIEHS, NIH, or the U.S. Government.
References
- Andersson PL, Öberg K, Örn U. Chemical characterization of brominated flame retardants and identification of structurally representative compounds. Environ Toxicol Chem. 2006;25:1275–82. doi: 10.1897/05-342r.1. [DOI] [PubMed] [Google Scholar]
- Arcadis EBRC Evaluation of data on flame retardants in consumer products. Final report to the European Commission. 2011:402. [Google Scholar]
- Babrauskas V, Blum A, Daley R, Birnbaum L. Flame retardants in furniture foam: benefits and risks; Fire Safety Science – Proceedings of the 10th International Symposium of the International Association for Fire Safety Science; London. 2012. [Google Scholar]
- Ballschmiter K. Zell. Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius Z Anal Chem. 1980;302:20–31. [Google Scholar]
- Ballschmiter K, Bacher R, Mennell A, Fisher R, Riehle U, Swerev M. The determination of chlorinated biphenyls, chlorinated dibenzodioxins and dibenzofurans by GC–MS. J High Resolut Chromatogr. 1992;15:260–70. [Google Scholar]
- Bergman Å . In: Alaee M, Reiner E, Clement R, editors. The abysmal failure of preventing human and environmental exposure to persistent brominated flame retardants: a brief historical review of BFRs; Commemorating 25 years of Dioxin Symposia; 2005.pp. 32–40. [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Gold MD, Ames BN, Jones FR, Hett EA, Dougherty RC, et al. Children absorb tris–BP flame retardant from sleepwear: urine contains the mutagenic metabolite, 2,3-dibromopropanol. Science. 1978;201:1020–3. doi: 10.1126/science.684422. [DOI] [PubMed] [Google Scholar]
- Covaci A, Gerecke AC, Law RJ, Voorspoels S, Kohler M, Heeb NV, et al. Hexabromocyclododecanes (HBCDs) in the environment and humans: a review. Environ Sci Technol. 2006;40:3679–88. doi: 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- Covaci A, Voorspoels S, Abdallah M, Geens T, Harrad S, Law R. Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J Chromatogr A. 2009;1219:346–63. doi: 10.1016/j.chroma.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Covaci A, Harrad S, Abdallah MA-E, Ali N, Law RJ, Herzke D, et al. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ Int. 2011;37:532–56. doi: 10.1016/j.envint.2010.11.007. [DOI] [PubMed] [Google Scholar]
- de Boer J, de Boer K, Boon JP. Polybrominated biphenyls and diphenylethers. In: Paasivirta J, editor. The handbook of environmental chemistry. Part K: new types of persistent halogenated compoundsHeidelberg. Springer-Verlag; Germany: 2000. pp. 61–95. [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- de Wit CA, Kierkegaard A, Ricklund N, Sellström U. Emerging brominated flame retardants in the environment. In: Eljarrat E, Barceló D, editors. The handbook of environmental chemistry. Brominated flame retardantsBerlin Heidelberg. Springer-Verlag; Germany: 2011. pp. 241–86. [Google Scholar]
- DiGangi J, Blum A, Bergman Å , de Wit CA, Lucas D, Mortimer D, et al. San Antonio statement on brominated and chlorinated flame retardants. Environ Health Perspect. 2010;118:A516–8. doi: 10.1289/ehp.1003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Silva K, Fernandes A, Rose M. Brominated organic micro-pollutants – igniting the flame retardant issue. Crit Rev Environ Sci Technol. 2004;34:141–207. [Google Scholar]
- EFRA. European Flame Retardants Association [last accessed on 12 December 2011];Market Statistics. 2007 http://www.ceficefra.com/Content/Default.asp?PageID=200.
- EFSA Scientific opinion on polybrominated biphenyls (PBBs) in food. EFSA J. 2010;8:1789. 151 pp. [Google Scholar]
- EFSA Scientific opinion on polybrominated diphenyl ethers (PBDEs) in food. EFSA J. 2011a;9:2156. 274 pp. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); scientific opinion on hexabromocyclododecanes (HBCDDs) in food. EFSA J. 2011b;9:2296. [Google Scholar]
- EFSA Scientific opinion on tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA J. 2011c;9:2477. doi: 10.2903/j.efsa.2024.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific opinion on brominated flame retardants (BFRs) in food: brominated phenols and their derivatives. EFSA J. 2012;10:2634. [Google Scholar]
- Eljarrat E, Barceleó D. Brominated flame retardants. Springer; New York: 2011. [Google Scholar]
- Fiedler H. Short-chain chlorinated paraffins: production, use and international regulations. In: De Boer J, editor. The handbook of environmental chemistry. Springer-Verlag; Chlorinated paraffinsBerlin Heidelberg, Germany: 2010. pp. 1–40. [Google Scholar]
- Gold MD, Blum A, Ames BN. Another flame retardant, tris–(1,3-dichloro-2-propyl)-phosphate, and its expected metabolites are mutagens. Science. 1978;200:785–7. doi: 10.1126/science.347576. [DOI] [PubMed] [Google Scholar]
- Harju M, Heimstad ES, Herzke D, Sandaanger T, Posner S, Wania F. Current state of knowledge and monitoring requirements: emerging “new” brominated flame retardants in flame retarded products and the environment. Norwegian Pollution Control Authority. 2009:1–114. [Google Scholar]
- Hindersinn RR. Historical aspects of polymer fire retardance. In: Nelson GL, editor. Fire and polymers hazard identification and prevention; American Chemical Society Symposium Series New York: American Chemical Society; 1990. [Google Scholar]
- Law RJ, Alaee M, Allchin CR, Boon JP, Lebeuf M, Lepom P, et al. Levels and trends of polybrominated diphenylethers (PBDEs) and other brominated flame retardants in wildlife. Environ Int. 2003;29:757–70. doi: 10.1016/S0160-4120(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, lepom P, et al. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Law RJ, Herzke D, Harrad S, Morris S, Bersuder P, Allchin CR. Levels and trends of HBCD and BDEs in the European and Asian environments, with some information for other BFRs. Chemosphere. 2008;73:223–41. doi: 10.1016/j.chemosphere.2008.02.066. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Klasson-Wehler E, Bergman A. The handbook of environmental chemistry volume 3K: anthropogenic compounds. Springer-Verlag; Berlin Heidelberg, Germany: 2000. Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls; pp. 315–59. [Google Scholar]
- Letcher RJ, Gebbink WA, Sonne C, Born EW, McKinney MA, Dietz R. Bioaccumulation and biotransformation of brominated and chlorinated contaminants and their metabolites in ringed seals (Pusa hispida) and polar bears (Ursus maritimus) from East Greenland. Environ Int. 2009;35:1118–24. doi: 10.1016/j.envint.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Nicholls CR, Allchin CR, Law RJ. Levels of short and medium chain length polychlorinated n-alkanes in environmental samples from selected industrial areas in England and Wales. Environ Pollut. 2001;114:415–30. doi: 10.1016/s0269-7491(00)00230-x. [DOI] [PubMed] [Google Scholar]
- OJ Off J Eur Union. 2003;L 37:19–23. 13.02.2003. [Google Scholar]
- OJ Off J Eur Union. 2008;C 116:2–3. 09.5.2008. [Google Scholar]
- Örn U, Bergman Å . An attempt to assess the present commercial production of brominated flame retardants; Third International Workshop on Brominated Flame Retardants, BFR 2004; Toronto, Canada. 2004.pp. 467–72. [Google Scholar]
- Papachlimitzou A, Barber JL, Losada S, Bersuder P, Law RJ. A review of the analysis of novel brominated flame retardants. J. Chromatogr. A. 2012;1219:15–28. doi: 10.1016/j.chroma.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AMCM, Everts JW, de Boer J, Boon JP. Polybrominated biphenyl and diphenylether flame retardants: analysis, toxicity and environmental occurrence. Rev Environ Contam Toxicol. 1995;141:1–26. doi: 10.1007/978-1-4612-2530-0_1. [DOI] [PubMed] [Google Scholar]
- State of California. Department of Consumer Affairs . Bureau of Home Furnishings and Thermal Insulation, 117. Technical Bulletins; Sacramento, California: 2000. p. 133. [Google Scholar]
- Sverko E, Tomy GT, Reiner EJ, Li Y-F, McCarry BE, Arnot JA, et al. Dechlorane plus and related compounds in the environment: a review. Environ Sci Technol. 2011;45:5088–98. doi: 10.1021/es2003028. [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–52. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- WHO/IPCS Environmental Health Criteria 162. Brominated diphenyl ethers. 1994:347. [Google Scholar]
- WHO/IPCS Environmental Health Criteria 172. Tetrabromobisphenol A and derivatives. 1995:139. [Google Scholar]
- WHO/IPCS Environmental Health Criteria 192. Flame retardants: a general introduction. 1997:133. [Google Scholar]
- Zennegg M. Identification of novel brominated flame retardants in new products of the Swiss market. Organohalogen Compd. 2011;73:1238–41. [Google Scholar]