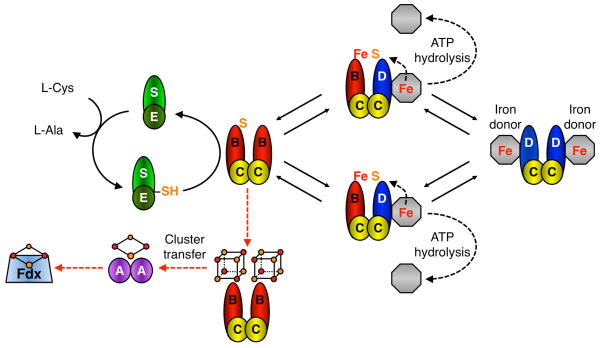
Scheme 1. Putative model of Suf-mediated Fe-S cluster assembly.
SufS releases sulfur from L-cysteine as a persulfide intermediate that is passed on to SufE and then SufB as part of the SufB2C2 complex [20]. The SufC2D2 complex may interact with an unidentified iron donor/iron source (grey octagon). SufB2C2 and SufC2D2 complexes interact to form two SufBC2D complexes where ATP hydrolysis by SufC is used to release iron from a donor or otherwise mobilize iron for cluster assembly (black dashed arrows) [21]. The complexes then dissociate to perform another round of sulfur and iron acquisition. SufD and SufC ATPase activity are required for in vivo iron acquisition although they may also play other roles in cluster assembly. Once a maximum of 2 × [4Fe-4S] clusters have formed, the terminal SufB2C2 scaffold may exit the cycle and, via an ATP-independent step, transfer clusters to Fe-S carrier proteins like SufA for further cluster trafficking to target enzymes like Fdx (red dashed arrows). All multi-protein complexes shown in Scheme 1 are known to form in vivo and/or in vitro (except for the putative interaction with the as yet unidentified iron donor) [6, 7, 22, 31, 45, 46].