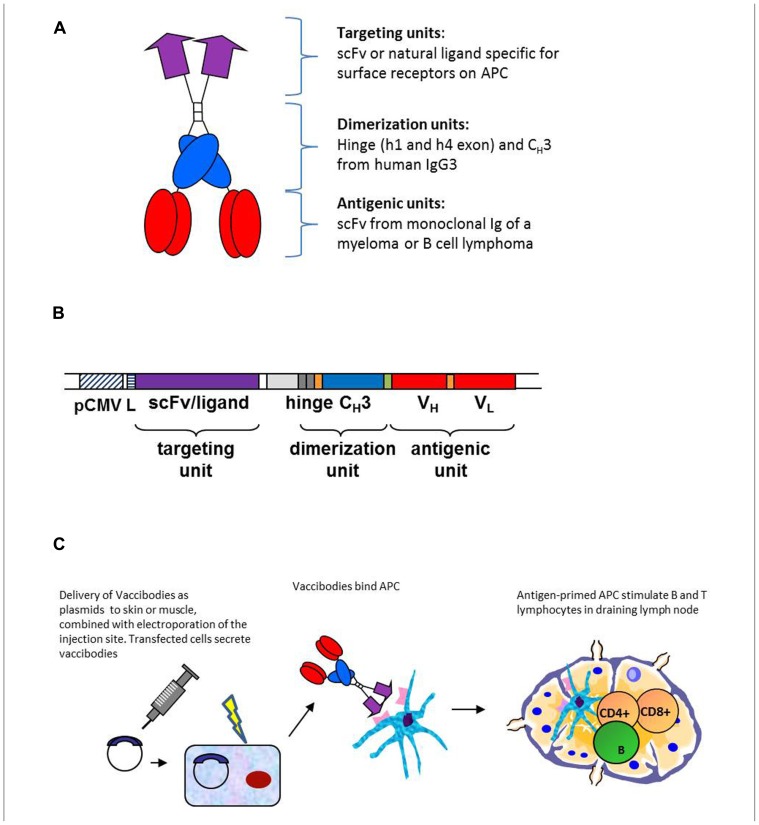
FIGURE 1.
Design, construction, and DNA delivery of APC-targeted vaccines. (A) The vaccine proteins are heterodimers. Each chain is composed of an N-terminal targeting unit [scFv(VL + VH) or chemokines, violet], that bind surface receptors on APC, a dimerization unit composed of a shortened human Ig hinge and CH3 domain (blue), and an antigenic unit corresponding to tumor-specific scFv from a B cell tumor (multiple myeloma, B cell lymphoma; red). As non-targeted controls we used vaccine molecule versions where the targeting unit was replaced with either scFv specific for the hapten NIP, or inactive (mutated) chemokine. (B) Gene construct. The targeting unit is inserted into the V cassette of the pLNOH2 vector. The dimerization unit, composed of h1 and h4 hinge exons and the CH3 exon of human IgG3 (blue), is linked to the antigenic unit (red) and inserted together in the C cassette of pLNOH2. The (G4S)3 linkers (orange) and the GLSGL linker (green) are indicated. The gene is expressed from a CMV promoter (hatched) and a leader sequence (striated) of the pLNOH2 vector (uncolored). Upstream of h1 is an intronic sequence (light gray) Reproduced with modifications from Mol. Ther. 13: 776–785, 2006, with permission from the publisher. (C) Transfected cells secrete vaccine proteins that target antigen presenting cells. The APC travels to the draining lymph node where it meets CD4+ T cells, B cells and CD8+ T cells.