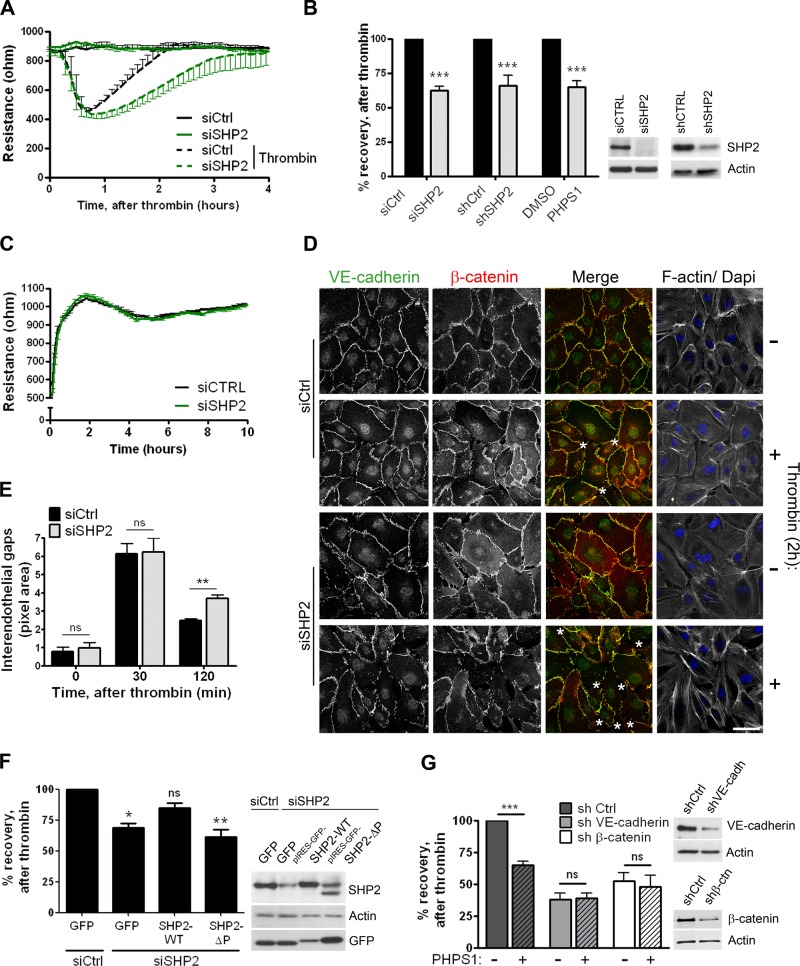
FIGURE 5:
SHP2 silencing and inhibition results in delayed cell–cell junction recovery after thrombin stimulation. (A) HUVECs were transfected with control (dark line) or SHP2 (green line) siRNA and grown to confluency on FN-coated electrode arrays. At time point 0, cells were incubated with (dashed line) or without (solid line) thrombin, and the electrical resistance was monitored in time by ECIS. Reduced SHP2 expression resulted in a delayed restoration of the endothelial monolayer TER after thrombin treatment. Representative graph is shown from one of five separate experiments, which were performed in quadruplicate. (B) HUVECs treated with SHP2 siRNA, shRNA, or SHP2 inhibitor PHPS1 were cultured on FN-coated ECIS electrode arrays and stimulated with thrombin. Bar graph, the percentage recovery of the endothelial monolayer resistance after thrombin at time points when control monolayers were completely restored. Both SHP2 silencing and SHP2 inhibition reduced recovery of the endothelial monolayer resistance. Data are mean ± SD of at least two independent experiments, which were performed in duplicate. Blot shows efficient SHP2 protein expression reduction in HUVECs that are transfected or transduced with either siRNA or shRNA against SHP2. (C) Cell spreading and monolayer formation of control and SHP2-depleted endothelial cells, analyzed by ECIS. Experiment was performed as described in A, except that the electrical resistance was monitored directly after cell seeding in serum-free medium. No difference in the electrical resistance during cell spreading and monolayer formation was measured between control and SHP2-deficient endothelial cells. Representative image is shown from one of four separate experiments, which were performed in quadruplicate. (D) HUVECs were grown on FN-coated glass covers, stimulated with thrombin for 2 h or left untreated, processed, and stained for VE-cadherin (green in merged image), β-catenin (red in merged image), and F-actin. Analysis of confocal image shows more interendothelial gaps (asterisks) in SHP2-depleted endothelial monolayers after 2 h of thrombin stimulation. Bar, 20 μm. (E) Quantification of data in D. Analysis of the size of interendothelial gaps showed increased gaps in SHP2-deficient cells 2 h after thrombin treatment. Experiment was carried out three times. Data are mean ± SEM. (F) HUVECs were treated with control or SHP2 siRNA and 2 d later infected with adenovirus containing pIRES-GFP-SHP2-WT or a phosphatase domain–deleted mutant (ΔP). Percentage recovery of the endothelial monolayer resistance after thrombin stimulation was determined as described in B. Reconstitution of SHP2-WT partially rescued recovery of the endothelial monolayer upon thrombin challenge, whereas SHP2-ΔP did not. Data are mean ± SD of three independent experiments, which were performed in duplicate. (G) VE-cadherin and β-catenin expression was reduced by shRNA. Endothelial cells were grown on FN-coated ECIS electrode arrays and treated with (dashed bars) or without (open bars) the SHP2 inhibitor PHPS1 for 20 h prior to thrombin stimulation. Bar graph shows that the effect of SHP2 inhibition on the restoration of the endothelial monolayer resistance is dependent on the expression of VE-cadherin and β-catenin. Blot shows reduction in VE-cadherin or β-catenin protein expression. Data are mean values of two independent experiments, which were performed in duplicate. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001.