Abstract
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death. To explore the potential of small interfering RNA (siRNA) therapy for NSCLC, we have developed anisamide-targeted LCP to efficiently deliver siRNA into the cytoplasm of sigma receptor-expressing NSCLC cells. Targeted LCP demonstrated a 9-fold higher siRNA delivery efficiency compared to non-targeted LCP in A549 cells in vitro. To simultaneously target multiple oncogenic mechanisms, we co-formulated three siRNA sequences targeting HDM2, c-myc and VEGF oncogenes, and investigated their efficacy of cell-killing in A549 and H460 cells in vitro. The results indicated that the pooled siRNA co-delivered by the targeted LCP could effectively and simultaneously knock down HDM2, c-myc and VEGF expressions and significantly inhibit tumor cell growth. After i.v. injection of mice bearing A549 xenografted tumor with Texas Red-labeled siRNA formulated in the targeted LCP, siRNA was successfully delivered to and concentrated in the tumor cells. Repeated intravenous injections of mice with pooled siRNA formulated in the targeted LCP significantly impaired NSCLC growth in vivo (p < 0.01) for both A549 and H460 tumors, demonstrating an ED50 for the treatment of ~0.2 mg/kg in A549 tumors. The enhanced anti-tumor activity is due to the fact that the silencing of HDM2/c-myc/VEGF could inhibit tumor proliferation and angiogenesis and also simultaneously induce tumor apoptosis. Our results demonstrate that the targeted LCP is a promising vector to deliver pooled siRNA into tumors and to achieve multiple target blocking. This is potentially a valid therapeutic modality in the gene therapy of human NSCLC.
Keywords: Nanoparticle, siRNA, NSCLC, Apoptosis, Angiogenesis, Drug delivery
Introduction
Approximately one third of all cancer-related deaths are due to lung cancer, which is also one of the most common cancers worldwide 1. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers. Surgical resection remains the single most consistent and successful option for cure. Unfortunately, close to 70% of patients with lung cancer present with locally advanced or metastatic disease at the time of diagnosis. The prognosis in the case of metastasis remains poor and chemotherapy provides only minimal gains in overall survival rates, resulting in a dismal 5-year survival rate of less than 15% 2, 3. Obviously, there is an urgent need to develop new treatment modalities for NSCLC.
Selective oncogene silencing, mediated by small interfering RNA (siRNA), has shown great promise for cancer therapy 4. However, practical use of siRNA in therapy is hindered by the fact that siRNA is highly susceptible to nuclease degradation and by its poor bioavailability 5. Efficient and biocompatible methods of delivery are needed to realize its full therapeutic potential. Recently, our group has developed a Calcium-Phosphate (CaP) based nanoparticles (NPs) named the Lipid/Calcium/Phosphate (LCP) 6. CaP is not only biocompatible and biodegradable 7, 8, it also binds nucleic acids with high affinity 9. Moreover, CaP rapidly dissolves at the acidic pH of the endosome, increasing the osmotic pressure and releasing the particle cargo into the cytoplasm 10. In LCP, the CaP core was stabilized with DOPA and further coated with a membrane containing cationic lipid. The final NPs demonstrate a hollow spherical structure and possess an asymmetric lipid bilayer at the surface. With the targeting ligand anisamide (AA), LCP could efficiently deliver siRNA to the sigma receptor-expressing tumor cells, and stimulate strong RNAi effect both in vitro and in vivo. Moreover, LCP showed less RES uptake and accumulated significantly in the tumor. Therefore, LCP delivery system shows potential as an attractive siRNA delivery system for treating cancer.
In the present study, we chose a range of pooled therapeutic siRNA (HDM2:c-myc:VEGF=1:1:1, weigh ratio) to target different molecular signaling facets of tumor development in established NSCLC xenografts. Accumulating evidence indicates that activation of oncogenes and growth factor signaling play a critical role in the oncogenesis of NSCLC 11. Simultaneously inhibiting multiple targets appears to be an effective approach for treating NSCLC. Conceptually, the approach is similar to the combinational chemotherapy, which is a common clinical practice. The HDM2 is an inactivator of p53 and plays a pivotal role in tumorigenesis, especially the regulation of apoptosis and cell cycle progression 12, 13. C-myc encodes a transcription factor that regulates gene networks controlling proliferation, metabolism, and ribosome biogenesis. Overexpression of the protooncogene c-myc has been implicated in the genesis of multiple human malignancies including NSCLC 14, 15. Production of VEGF, up-regulated in NSCLC tumors, is associated with angiogenesis and poor prognosis 16, 17. Here we report the highly efficacious therapeutic activity of pooled HDM2/c-myc/VEGF siRNA delivered by LCP in two different NSCLC models.
Materials and methods
Materials
1,2-distearoryl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol-2000) ammonium salt (DSPE-PEG2000), dioleoylphosphatydic acid (DOPA) and 1, 2-dioleoyl-3-trimethylammonium-propane chloride salt (DOTAP) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). DSPE-PEG-anisamide (AA) was synthesized in our lab as described previously 18. Fluorescein (FAM) labeled double-stranded oligonucleotide (oligo), with the same sequence of siRNA, was provided by Integrated DNA Technologies (Coralville, IA). Other chemicals were obtained from Sigma-Aldrich (St. Louis, MO) without further purification.
Mouse monoclonal primary antibodies against HDM2, c-myc, GAPDH, and secondary antibodies conjugated with horseradish peroxidase (goat anti-mouse IgG HRP) were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Quantikine VEGF ELISA kit was purchased from R&D Systems (Minneapolis, MN).
Cell lines
Human lung large cell carcinoma cell line H460 and human lung adenocarcinoma cell line A549 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). All cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 20 mM of l-glutamine, 100 U/mL of penicillin G sodium, and 100 mg/mL of streptomycin at 37°C in an atmosphere of 5% CO2 and 95% air.
siRNA
HDM2 siRNA (target sequence: 5′-AAG CCA UUG CUU UUG AAG UUA-3′), c-myc siRNA (target sequence: 5′-AAC AGA AAU GUC CUG AGC AAU-3′), VEGF siRNA (target sequence: 5′-ACC UCA CCA AGG CCA GCA C-3′), control siRNA (target sequence: 5′-AAU UCU CCG AAC GUG TCA CGU-3′) and Texas Red-labeled control siRNA were purchased from Dharmacon (Lafayette, CO) in unprotected, desalted, annealed form. All sequences were adopted from published studies 19–21.
Preparation of siRNA containing LCP
LCP was prepared as described previously with slight modifications 6. Briefly, 300 μL of 2.5 M CaCl2 with 12 μg of siRNA was dispersed in a 20 mL oil phase (cyclohexane/lgepal CO-520 (71/29, v/v)) to form a well-dispersed water-in-oil reverse micro-emulsion. The phosphate micro-emulsion was prepared by dispersing 300 μL of 12.5 mM Na2HPO4 (pH=9.0) in a separate 20 mL oil phase. 100 μL (20 mM) DOPA in chloroform was added to the phosphate solution. After mixing the above two micro-emulsions for 20 min, 40 mL ethanol was added to the combined solution and the mixture was centrifuged at 12,000 g for 20 min. After 3 cycles of ethanol wash, the CaP core pellets were dissolved in 2 mL chloroform and stored in a glass vial. To prepare the final LCP, 500 μL of CaP core was mixed with 75 μL of 10 mM DOTAP, 10 mM cholesterol, 3 mM DSPE-PEG-2000 and 3 mM DSPE-PEG-AA. After evaporating the chloroform, the residual lipid was hydrated in 200 μL of 5% glucose (GS) to form LCP. Zeta potential and particle size of the final LCP were measured by a Malvern ZetaSizer Nano series (Westborough, MA). The trapping efficiency of siRNA in LCP was determined as described previously using Texas Red-labeled siRNA 10. Transmission electron microscope (TEM) images of LCP were acquired using JEOL 100CX II TEM (JEOL, Japan).
In vitro cellular uptake
A549 cells (1 × 105 per well) were seeded in 12-well plates (Corning Inc., Corning, NY) with cover glass for 12 h before the experiment. Cells were treated with different formulations at a concentration of 100 nM FAM-labeled oligo in serum containing medium at 37 °C for 4 h. After washing twice with PBS, cells were fixed with 4% paraformaldehyde in PBS at room temperature for 10 min, counterstained with DAPI (Vector Lab, Burlingame, CA), and imaged with a florescence microscope. To quantify the in vitro uptake efficiency, cells were lysed with 300 mu;L lysis buffer (0.3% Triton X-100 in PBS) at 37 °C for 0.5 h. Fluorescence intensity of the 100 μL cell lysate was determined by a plate reader at an excitation wavelength of 485 nm and an emission wavelength of 535 nm (PLATE CHAMELEON Multilabel Detection Platform, Bioscan Inc., Washington, DC). For the free ligand competition study, cells were co-incubated with 50 μM haloperidol with different formulations.
Quantitative RT-PCR
A549 cells (2×105 per well) were seeded in 6-well plates (Corning Inc., Corning, NY) 12 h before treatment. Cells were treated with different LCP at a concentration of 33.3 nM for single siRNA or 100 nM for pooled siRNA (HDM2:c-myc:VEGF = 1:1:1, weight ratio) formulated in the targeted LCP at 37°C for 24 h. Total cell RNA was extracted with an RNeasy® Mini Kit (Qiagen, Valencia, CA), then individual cDNAs were synthesized with a SuperScript® II reverse transcriptase assay (Invitrogen, Carlsbad, CA). Real-time quantitative PCR (qPCR) was performed with a SYBR® GreenER™ qPCR SuperMix Universal kit (Invitrogen, Carlsbad, CA). Reactions were run with a standard cycling program: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min, on an AB7500 real-time PCR system (Applied Biosystems, Foster City, CA). The primer pairs for detecting the mRNA are listed in the Supplementary Table S1. One day after the last treatment, the mRNA levels of HDM2, c-myc and VEGF in the H460 tumor tissue were also analyzed by using qRT-PCR.
Colony formation assay
H460 cells (2 × 105) were treated with 100 nM pooled siRNA formulated in targeted or non-targeted LCP. Control siRNA formulated in targeted LCP served as a control. Twenty-four h after transfection, cells were plated in 6-well plates for 10 days. Staining was performed using 0.1% crystal violet in PBS for 30 min with shaking at room temperature. Colonies were counted after removing stain and washing cells with water.
Cell viability analysis by MTT assay
1 × 104 H460 or A549 cells were seeded in 200 μL culture medium per well into a 96-well plate. When cultured to 70% confluence, cells were treated with different formulations at a concentration of 100 nM for pooled siRNA (HDM2:c-myc:VEGF = 1:1:1, weight ratio), or left untreated. After incubating the cells for 48 h, MTT assay was carried out to evaluate the cell viability. Untreated cells served as the indicator of 100% cell viability. The absorbance was measured at 570 nm using a microplate reader.
Apoptosis analysis by flow cytometry
Apoptotic cells were visualized using an Annexin V-FITC apoptosis detection kit (BD Biosciences, San Jose, CA) according to the manufacturer’s protocol. Briefly, cells were harvested 48 h after transfection, washed twice with PBS, and re-suspended in 500 μL of Annexin V binding buffer. Two microliters of FITC-conjugated Annexin V was added to the cell solutions, followed by the addition of 5 μL of propidium iodide (PI). After incubation for 5 min at room temperature in the dark, samples were immediately analyzed using a FAC-SCalibur flow cytometer (BD Biosciences, San Jose, USA). Data from approximately 1 × 104 cells were analyzed by using the CELLQuest software (BD Biosciences, San Jose, USA).
Lung cancer xenograft and treatment
All animal experiments were performed in accordance with and approved by the Institutional Animal Care and Use Committee at the University of North Carolina. Cells (5 × 106) in 100 μL of PBS were injected s.c. in the lower back region of female nude mice. When tumor size reached approximately 50–100 mm3, animals were randomly divided into four groups and treated by tail vein injection with control siRNA formulated in targeted LCP (0.36 mg/kg), pooled siRNA (HDM2/c-myc/VEFG = 1:1:1, weight ratio, 0.36 mg/kg) formulated in non-targeted LCP, or pooled siRNA formulated in targeted LCP. Injections of 100 μL of 5% glucose (GS) were carried out at the same time points in the control group. Tumor-bearing mice were treated every 3 days for a total of five times for H460 xenograft model and every 5 days for a total of six times for A549 xenograft model. Tumor size was determined by caliper measurement. One day after the last injection, H460 tumors and organs were collected, dissected, and fixed in 10% formalin for histology.
Tissue distribution study
Nude mice bearing A549 xenograft with tumor size of approximately 1 cm2 were i.v. injected with Texas Red-labeled siRNA in different formulations (0.36 mg/kg). Four h later, mice were sacrificed and tissues were collected and imaged by the Kodak In-Vivo Imaging System Fx Pro (Kodak, Rochester, NY). Frozen sections of tumor tissue with a thickness of 6-μm were also prepared. Sections were mounted with the DAPI-containing medium (Vectashield®, Vector Laboratories Inc., Burlingame, CA) and imaged using a Leica SP2 confocal microscope.
Dose response study
In dose-response experiments, female nude mice bearing A549 NSCLC xenograft were given i.v. injections of 90–720 μg/kg pooled siRNA formulated in the targeted LCP every four days. Mice were sacrificed after 5 injections.
Immunohistochemistry
Tumor cell proliferation was determined by immunostaining H460 tumor tissues with monoclonal mouse PCNA antibody (Santa Cruz Biotechnology, Santa Cruz, CA) as described previously 22. Cell nuclei were counterstained with hematoxylin. The cell proliferation index was determined by the percentage of PCNA-positive cells in 10 high-powered fields at 100× magnification.
Terminal deoxyribonucleotide transferase (TdT)–mediated nick-end labeling (TUNEL) staining was performed to detect apoptotic cells in H460 tumor tissues by using a DeadEndTM Fluorometric TUNEL System (Promega, Madison, WI), following the manufacturer’s protocol. Cell nuclei with dark green fluorescent staining were defined as TUNEL-positive nuclei. TUNEL-positive nuclei were monitored using a fluorescence microscope. To quantify TUNEL-positive cells, the number of green-fluorescence–positive cells was counted in 10 random fields at 200× magnification.
CD31 immunohistochemistry was done on fresh frozen sections of H460 tumor tissue. After fixation with cold acetone for 20 min, samples were incubated with 1:50 dilution of rat monoclonal anti-mouse CD31 antibody (BD Pharmingen, San Diego, CA) at 4 °C for 1 h followed by incubation with HRP-conjugated goat anti-rat IgG antibody (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min. Visualization was achieved with a peroxidase detection kit (Pierce, Rockland, IL). Microvessel density was quantified by the number of lumen-like structures adjacent to CD31-positive endothelial cells. All specimens were examined with a Nikon light microscope (Nikon Corp., Tokyo, Japan).
Toxicity and pathology studies
For liver and renal function experiments, mouse serum was collected 24 h after the final treatment. The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and blood urea nitrogen (BUN) in serum were analyzed by the Animal Clinical Chemistry and Gene Expression Laboratories at the University of North Carolina at Chapel Hill. Major mouse organs were also collected after the treatments, fixed in formalin, and processed for routine H&E staining using standard methods. Images were collected using a Nikon light microscope.
Statistical analysis
Data are presented as mean values ± SD. The statistical significance was determined by using one-way ANOVA. P values < 0.05 were considered significant.
Results
Characterization of LCP
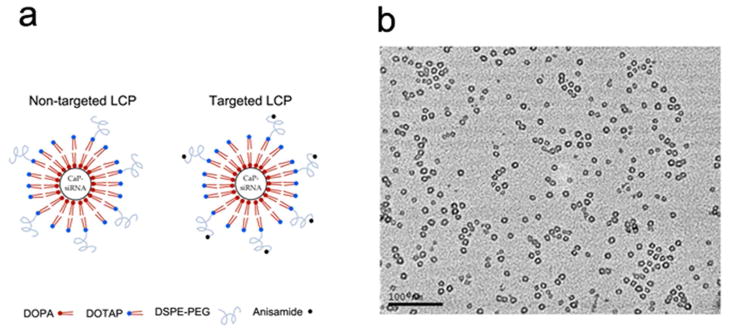
The structure of the non-targeted LCP and targeted LCP is illustrated in Fig. 1a. In the LCP, the CaP-siRNA core was stablized with DOPA, coated with DOTAP and further grafted with DSPE-PEG or DSPE-PEG-AA. The characteristics of the LCP are summarized in Table 1. The particle sizes of the non-targeted LCP and targeted LCP were similar to each other. Targeted LCP showed a slight increase in the zeta potential compared to the non-targeted LCP due to the positively charged anisamide ligand. TEM was also employed to examine the morphology of the final LCP. Since the lipid bilayer is electron transparent, only the CaP cores of the NPs were imaged, and were detected as hollow, spherical structures (Fig. 1b). Additionally, neither the non-targeted nor the targeted LCP formed aggregates in 50% serum (data not shown). The siRNA trapping efficiency of the LCP was determined to be ~ 91% using Texas Red-labeled siRNA.
Figure 1.
(a) Illustration of non-targeted and targeted LCP; (b) TEM images of LCP coated with DOTAP and DSPE-PEG.
Table 1.
Characterization of LCP
| Non-targeted LCP | Targeted LCP | |
|---|---|---|
| Particle size (nm) | 36.1 ± 4.8 | 38.6 ± 3.6 |
| Zeta potential (mV) | 25.5 ± 2.1 | 29.1 ± 1.3 |
The particle size and zeta potential of non-targeted and targeted LCP were measured using a Zetasizer. Data are representative data from repeated measures of 3–4 samples.
In vitro cellular uptake and gene silencing analysis
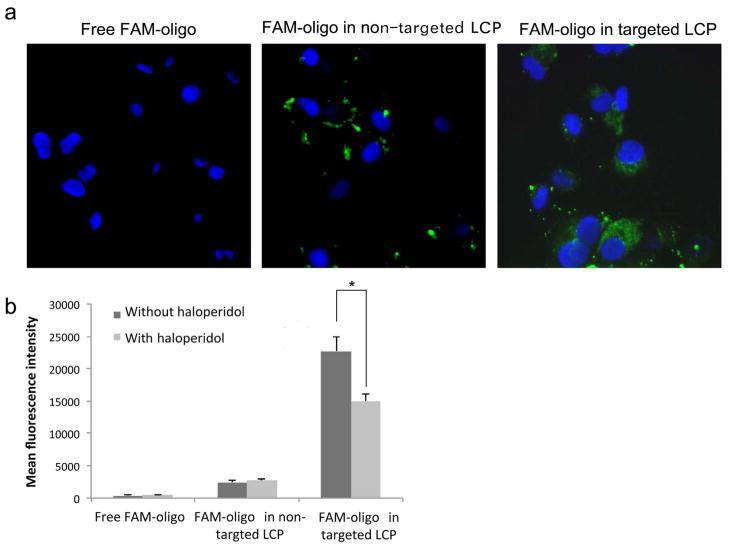
Previous reports have shown that sigma receptors are overexpressed in a variety of human tumors including NSCLC cells, which makes the sigma ligand a potentially interesting ligand for targeting drugs to such tumors 18. In the present study, we grafted the LCP with PEG and AA ligand on the surface to target sigma receptors that were overexpressed on the NSCLC cancer cells. To investigate the delivery efficiency of the LCP, we performed the cellular uptake study by using FAM-labeled oligo as a model for siRNA. As shown in Fig. 2a, FAM-labeled oligo was evenly spread throughout the cytoplasm of A549 cells when carried in AA targeted LCP, but not when formulated in non-targeted LCP. To quantify the cellular uptake, A549 cells treated with different LCP formulations were lysed, and the fluorescence intensity of the cell lysate was measured. As shown in Fig. 2b, cell lysate from cells treated with free FAM-oligo showed background fluorescence. The delivery efficiency of non-targeted LCP was low and was not affected by haloperidol, a known agonist for sigma receptors. However, targeted LCP demonstrated significantly increased siRNA delivery efficiency compared to the non-targeted LCP. The addition of haloperidol significantly reduced the delivery efficiency of targeted LCP (p < 0.05). Thus, the reduction of siRNA delivery after the introduction of haloperidol, a competitive agonist, demonstrates that the targeted LCP was able to successfully deliver siRNA in a sigma receptor-mediated process.
Figure 2.
Cellular uptake of siRNA in vitro. (a) Fluorescence photographs of cultured A549 cells after treatment with 5′-FAM-labeled oligo in non-targeted or targeted LCP for 4 h. (b) Quantitative measurement of mean fluorescence intensity of cell lysate from A549 cells treated with 5′-FAM-labeled oligo formulated in non-targeted or targeted LCP. A549 cells were incubated at 37 °C for 4 h in the absence or presence of 50 μM haloperidol. Columns, mean (n = 3); bars, SD. *, p < 0.05.
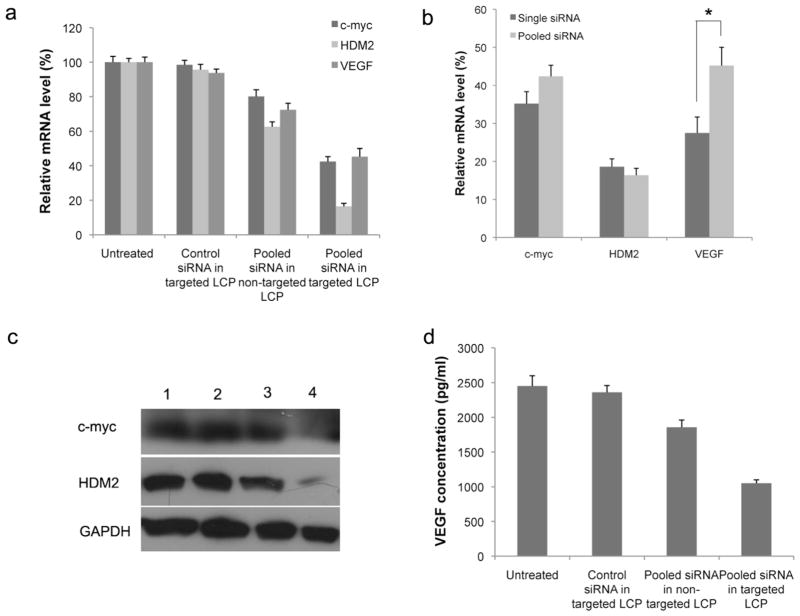
Next, we formulated the pooled siRNA (HDM2/c-myc/VEGF=1:1:1) in targeted or non-targeted LCP and tested the gene silencing effect of the formulation in A549 tumor cells. Cellular mRNA was extracted and analyzed using qPCR 48 h post transfection. The results indicated that pooled siRNA simultaneously inhibited HDM2, c-myc and VEGF expression, demonstrating silencing efficiencies of 87.6%, 57.6% and 54.8% for HDM2, c-myc and VEGF, respectively (Fig. 3a). We also quantified the RNA interference activity of a single sequence and pooled sequences delivered by the targeted LCP. We found a sequence competition effect, with the pooled siRNA formulation showing significantly reduced VEGF gene silencing activity (Fig. 3b, p < 0.05). This competitive effect and silencing reduction, however, was not observed in the silencing of HDM2 and c-myc genes when compared to the single siRNA formulations. We also analyzed the RNAi activity in the protein levels by western blotting and ELISA analysis. As shown in Fig. 3c and Fig. 3d, pooled siRNA in the targeted NPs was able to silence HDM2, c-myc, and VEGF in the A549 cells when delivered at a concentration of 100 nM, whereas treatment with the pooled siRNA in non-targeted LCP resulted in only a slight gene silencing effect when compared with treatment with control siRNA in targeted LCP.
Figure 3.
In vitro oncogene silencing. (a) Relative mRNA level after transfection of A549 cells with pooled siRNA in targeted LCP. (b) Comparison of each oncogene silencing efficiency for single siRNA formulated LCP versus pooled siRNA formulated LCP. (c) Western blot analysis of Hdm2 and c-myc after treatment of A549 cells with siRNA in different LCP formulations: 1) untreated; 2) control siRNA in targeted LCP; 3) pooled siRNA in non-targeted LCP; 4) pooled siRNA in targeted LCP. (d) ELISA analysis of VEGF in the A549 cell supernatant after treatment with pooled siRNA in different LCP formulations. Columns, mean (n = 3, in triplicate); bars, SD. *, p < 0.05.
Pooled siRNA formulated in targeted LCP inhibit tumor cell growth in vitro
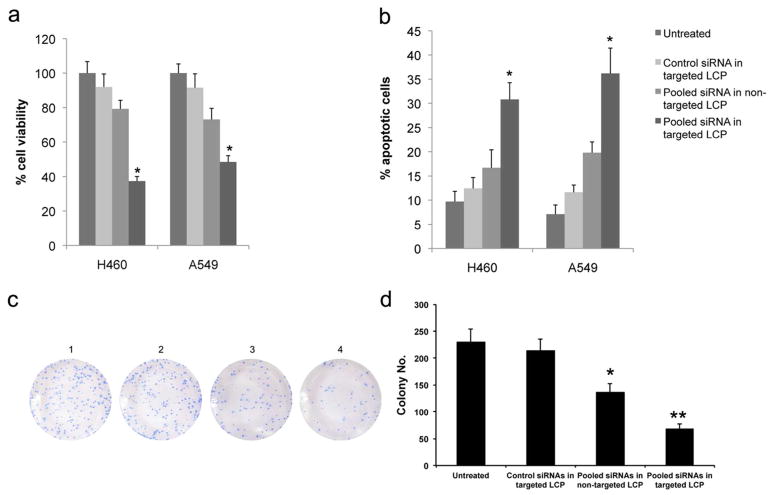
We next determined the influence of HDM2, c-myc and VEGF down-regulation on A549 and H460 NSCLC cells in vitro. Cellular proliferation was monitored by MTT assay. Compared with untransfected cells and control groups, cellular proliferation was significantly inhibited in cells treated with the pooled siRNA formulated in the targeted LCP (Fig. 4a, p < 0.05). Flow cytometry was used to assess the number of apoptotic cells. The data revealed a significant increase of apoptosis in the targeted LCP group (Fig. 4b, p < 0.05). These findings suggest that the pooled siRNA formulated in targeted LCP could significantly inhibit cell proliferation, and induce apoptosis in cancer cells in vitro. In contrast, pooled siRNA formulated in non-targeted LCP only showed a minimal effect on cell proliferation inhibition and apoptotic induction (p > 0.05). We also performed a colony formation assay to test the effect of pooled siRNA in different formulations (Fig. 4c). The results show that pooled siRNA in targeted LCP significantly inhibited colony formation of H460 cells (Fig. 4d, p < 0.01). The non-targeted group also showed significant inhibition of the tumor cell colony formation (p < 0.05), but the degree of inhibition was less than that of the targeted formulation.
Figure 4.
In vitro oncogenes silencing inhibits NSCLC proliferation and induces apoptosis. (a) Cell viability was measured by MTT assay after treatment with different LCP for 48 h. (b) Histogram showing the quantification of apoptotic cells treated with different LCP for 48 h. (c) Representative colony formation assay of NSCLC cells treated with different formulation. 1) untreated; 2) control siRNA in targeted LCP; 3) pooled siRNA in non-targeted LCP; 4) pooled siRNA in targeted LCP. All colonies were stained with crystal violet. (d) Histogram showing the quantification of colony formation efficiency. Columns, mean (n = 3); bars, SD. *, p < 0.05; **p < 0.01.
Uptake of siRNA in vivo
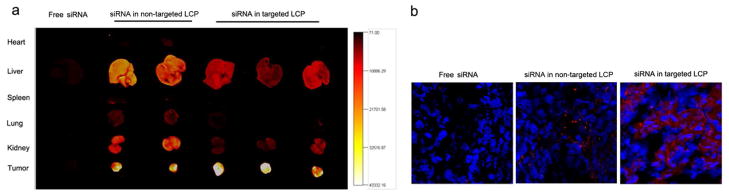
Texas Red-labeled siRNA in different formualtions was i.v. injected into A549 xenograft mice, and the fluorescence distribution in the major organs was detected after 4 h. As shown in Fig. 5a, fluorescence signals were barely detected in organs collected from the mice treated with free labeled siRNA. The Texas Red-labeled siRNA accumulated in the liver, kidney and tumor with little selectivity when formulated in the non-targeted LCP. In comparison, the tumor showed the strongest signal, and all other major organs showed only background to moderate signals in mice treated with the targeted LCP. Fig. 5b also shows negligible accumulation of free Texas Red-siRNA in the tumor examined by fluorescence microscopy of frozen tumor sections. The tumor tissue fluorescence signal was weaker and more heterogeneous in the non-targeted LCP group when compared with the targeted LCP group. Therefore, treatment of mice with labeled siRNA formulated in the targeted LCP resulted in significantly increased tumor penetration and uptake.
Figure 5.
In vivo cellular uptake of siRNA. (a) In vivo biodistribution of Texas Red-siRNA formulated in non-targeted LCP or targeted LCP; (b) Tumor uptake of Texas Red-labeled siRNA in the non-targeted LCP or targeted LCP. Magnification = ×400.
Tumor growth inhibition by LCP containing pooled siRNA
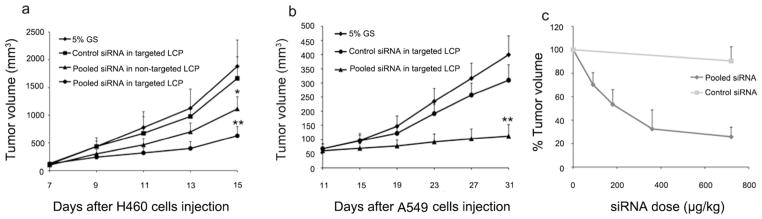
The data obtained in vitro showed that pooled siRNA formulated in LCP could enable a significant inhibitory effect on tumor cell survival. In order to test this effect in vivo, nude mice were inoculated with H460 or A546 NSCLC cells and the xenografts were allowed to grow to a volume of 50–100 mm3 before treatment. The mice were randomly assigned to 4 treatment groups: 1) pooled siRNA in targeted LCP; 2) pooled siRNA in non-targeted LCP; 3) control siRNA in targeted LCP; and 4) 5% glucose (GS), respectively. As shown in Fig. 6a and Fig. 6b, treatment with pooled siRNA in targeted LCP significantly reduced tumor growth (p < 0.01), whereas control siRNA in targeted LCP had little effect. The growth delay of tumors in mice injected with pooled siRNA in targeted LCP continued until the day of sacrifice, while tumors of mice injected with 5% GS and control siRNA in targeted LCP grew persistently. Pooled siRNA in non-targeted LCP also significantly inhibited the H460 tumor growth compared to control groups (p < 0.05), but the degree of inhibition was less than that of the targeted NPs. The results suggest that multiple dosing might compensate for the relatively poor uptake efficiency of the non-targeted NPs. The activity of tumor growth inhibition by the pooled siRNA in LCP was dose dependent. The estimated ED50 was ~200 μg/kg for the pooled siRNA formulated in the targeted LCP (Fig. 6c).
Figure 6.
In vivo oncogenes silencing inhibits NSCLC tumor growth. (a) Tumor growth curve of H460 subcutaneous model. (b) Tumor growth curve of A549 subcutaneous model. (c) Dose response curve on A549 subcutaneous model. ED50=205 μg/kg. Data = mean ± SD, n = 5. *, p < 0.05; **, p < 0.01 comparing to the 5% GS group. H460 tumors were treated every 3 day starting at day 7 for 5 times and A549 tumors was treated every 5 days starting at day 11 for 6 times.
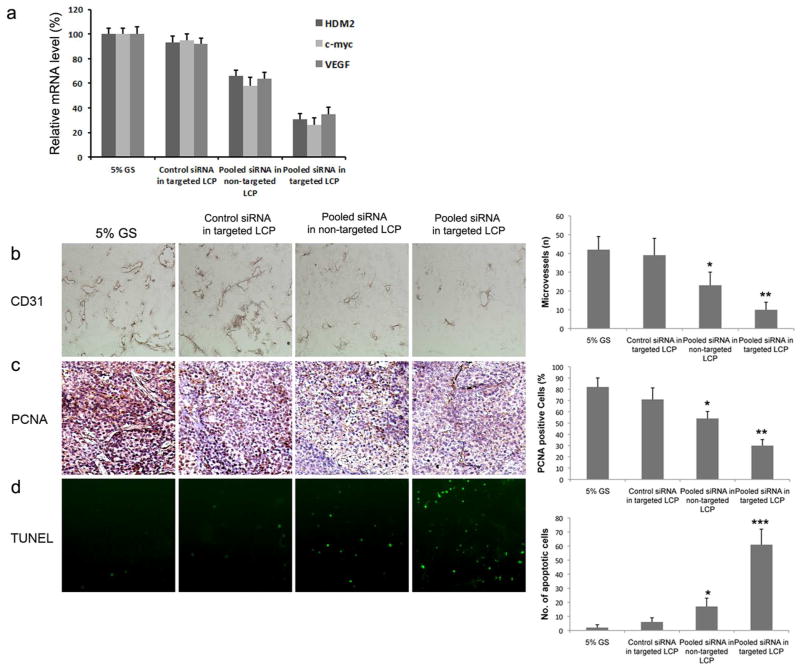
In vivo antitumor mechanism
To elucidate the mechanism underlining the antitumor effect of the targeted LCP, gene silencing activity in H460 tumor tissues was measured using qRT-PCR one day after the last treatment. As shown in Fig. 7a, pooled siRNA formulated in the targeted LCP could simultaneously silence HDM2, c-myc, and VEGF in the H460 xenografts, whereas control siRNA in targeted LCP showed little effect. Pooled siRNA formulated in the non-targted LCP also showed significant gene silencing when compared with the 5% GS group, but the degree of silencing was less than that of the targeted LCP. To determine the biological effects of treatment with pooled siRNA in targeted LCP, H460 tumors harvested from mice following therapy were subjected to imaging measurements for cell proliferation (PCNA), apoptosis (TUNEL), and mean vessel density (CD31). Treatment with pooled siRNA in non-targeted LCP resulted in a modest 45% decrease in MVD compared to the 5% GS control (Fig. 7b, p < 0.05), but the greatest decrease in MVD was noted in the targeted LCP group (76% decrease, p < 0.01). There was a 36% reduction in the mean number of PCNA-positive cells in non-targeted LCP treated mice compared to the control (Fig. 7c, p < 0.05). The targeted LCP treatment group produced the largest reduction in PCNA-positive cells (63%, p < 0.01). We further performed in situ TUNEL assay to evaluate the influence of oncogene knockdown on apoptosis of tumor cells. We observed an elevated apoptosis rate in tumors of mice injected with pooled siRNA formulated in targeted LCP compared with that of tumors from mice injected with control siRNA and 5% glucose (Fig. 7d, p < 0.001). Therefore, the results demonstrated that the targeted LCP could efficiently deliver pooled siRNA to the NSCLC tumor, leading to reduced cell proliferation, angiogenesis and increased apoptosis in vivo, which resulted in a persistent inhibition of tumor growth.
Figure 7.
Effects of oncogene silencing on the cell apoptosis, angiogenesis and cell proliferation in vivo. (a) Relative HDM2, c-my and VEGF mRNA level in the H460 tumor tissues. Columns, mean (n = 5); bars, SD; (b) CD31 staining of microvessels in H460 tumor treated with different formulations (original magnification × 200). (c) PCNA-positive nuclei in H460 tumor treated with different formulations (original magnification × 200). (d) TUNEL assay of H460 tumor treated with different formulations (original magnification × 100). Quantitation of the image data is shown in the histograms on the right. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Toxicity assay
Levels of secreted liver enzymes (AST and ALT), and BUN were all unchanged after a long-term treatment (6 times) of H460 tumor bearing mice with pooled siRNA in targeted LCP, indicating a lack of damage to the liver or kidneys (Table 2). We also performed a pathologic examination of the major organs (lung, liver, kidney, spleen, heart) from the mice that received long-term treatments by H&E staining (data not shown). No organ damage was observed in samples from mice treated with the formulated LCP. Additionally, the body weight of experimental mice did not significantly decrease during treatment at the therapeutic dose (data not shown).
Table 2.
Serum levels of ALT, AST and BUN after long-term treatment with therapeutic siRNA in the targeted LCP
| ALT (U/L) | AST (U/L) | BUN (mg/dL) | |
|---|---|---|---|
| Untreated group | 55.5 ± 9.12 | 140.5 ± 10.61 | 16 ± 2.83 |
| Therapeutic siRNA in targeted LCP treated group | 58.33 ±17.5 | 149.33 ± 24.91 | 20 ± 5.29 |
Measurements were carried out with the aim of evaluating the potential long-term toxicity of pooled siRNA in the targeted LCP. Data = mean ± SD (n = 5).
Discussion
Although progress has been made in the past 10 years in the management of patients with NSCLC, the 5-year survival rate has not improved substantially 23. Advances in the knowledge of tumor biology and the mechanisms of oncogenesis have revealed several potential molecular targets for NSCLC treatment 24. We show here that a novel receptor-targeted delivery system (LCP) loaded with pooled siRNA targeting HDM2, c-myc and VEGF oncogenes led to potent antitumor efficacy in NSCLC. HDM2 is essential for p53 function 12, 13. C-myc controls several proliferation pathways in the tumor cells 14, 15. VEGF is a well-established angiogenesis factor that is essential for tumor growth 25. Simultaneous down-regulation of these three oncogenic elements by RNAi is predictively effective in stimulating tumor cell apoptosis and limiting the tumor growth as shown by the current study.
CaP-based composite NPs have been recently developed for siRNA delivery 6, 26, 27. CaP is an attractive nanoparticle for siRNA delivery because the CaP can efficiently dissolve in the low pH microenvironment of the endosome, increase the osmotic pressure, and cause the endosome to swell and burst for a more consistent release of entrapped siRNA into the cytoplasm 10. Moreover, CaP is biodegradable, biocompatible, and has low immunogenicity 7, 9, 28. However, uncoated CaP NPs demonstrate limited transfection efficiency and reproducibility because of relatively poor stability. They are not commonly used for in vivo nucleic acid delivery 29–31. In the present study, we have utilized a new, lipid membrane coated CaP NPs, i.e. LCP 6, to deliver siRNA in mouse NSCLC models. In the LCP formulation, the CaP core, stabilized with anionic lipid (DOPA), was prepared by using reverse microemulsion synthetic approach and further coated with cationic lipid (DOTAP: Cholesterol=1:1). To prolong the in vivo circulation time and improve the cellular uptake, the final LCP was PEGylated with DSPE-PEG2000 or DSPE-PEG-AA. The LCP were ~40 nm in diameter, which is a suitable size to be fit into a clathrin coated pit and internalized by receptor-mediated endocytosis 4. The zeta potentials were around 25 mV for LCP. It has been shown that the hydrophilic and neutral PEG molecule can screen the positive charge by DOTAP/cholesterol lipid bilayer and thereby lower the surface charge. Thus, the low zeta potential was an indication of a high degree of PEGylation and vice versa 32. PEGylation on the nanoparticle surface could also maintain colloidal stability when redispersed after drying 33. The TEM photograph revealed that LCP were well dispersed with a spherical morphology (Fig. 1b). The cellular uptake study showed that the FAM-labeled oligo was evenly distributed throughout the cytoplasm of A549 cells when carried in AA targeted LCP (Fig. 2a). The reduction of siRNA delivery after the introduction of haloperidol, a competitive agonist, demonstrated that the targeted LCP were able to successfully deliver siRNA in a sigma receptor-mediated process (Fig. 2b). Moreover, after i.v. injection of labeled siRNA formulated in the targeted LCP, most of the injected siRNA accumulated in the tumor tissue (Fig. 5a). The distribution of Texas Red-siRNA in the tumor was heterogeneous (Fig. 5b). This result was consistent with the previous data acquired from the LPD system: densely PEGylated lipid nanoparticles exhibited higher passive targeting to the tumor with leaky vessels due to the extended blood circulation time 32. Furthermore, after the LCP penetrates into the tumor mass, the targeting ligand on the surface can then facilitate cellular internalization, after which the pH-sensitive CaP core can disassemble in the acidic endosome and efficiently release the cargo nucleic acid into the cytoplasm. Since the RISC complex where RNAi takes place resides in the cytoplasm, this is the final destination of the delivered siRNA.
It has been reported that co-delivery of multiple siRNA sequences could reduce the efficacy of RNA silencing due to the competition of siRNA for incorporation into RISC. We did find a sequence competition effect, with the pooled siRNA formulations showing significantly reduced VEGF gene silencing activity (Fig. 3b, p < 0.05). However, this competitive effect and silencing reduction was not observed in the silencing of HDM2 and c-myc genes when compared to the single siRNA formulations. Overall, the competitive effect was minimal, and significant gene knockdown (50–90%) was observed for all the gene targets when 100 nM pooled siRNA was used. Moreover, after transfection with pooled siRNA formulated in the targeted LCP, cell proliferation was significantly inhibited and apoptosis was significantly enhanced in both A549 and H460 cells (Fig. 4a, b, p < 0.05).
The most remarkable results obtained using pooled siRNA formulated in the targeted LCP are those shown in vivo. Tumor growth is delayed in mice treated with pooled siRNA formulated in the targeted LCP. Microvascular density, measured by CD31 immunostaining, is significantly lower than in control tumors, providing proof of the true anti-angiogenic activity of pooled siRNA. Moreover, to further investigate the anti-tumor mechanism, we evaluated the effect of pooled siRNA on cell apoptosis and proliferation by TUNEL and PCNA immunostaining, respectively. Our data revealed that pooled siRNA formulated in targeted LCP could increase apoptosis of tumor cells (p < 0.001) and inhibit tumor cell proliferation (p < 0.01). We have shown earlier that by using the pH sensitive CaP core, LCP could enhance the efficiency of siRNA release 6. Consistent with this finding, the current study showed that ED50 of the targeted NPs was relatively low (ED50 = 200 μg/kg). We also found that pooled siRNA in non-targeted LCP could delay the tumor growth compared with the control groups (p < 0.05). The results suggested that multiple dosing might compensate for the relatively poor uptake efficiency of the non-targeted NPs. Furthermore, the pooled siRNA formulated in targeted LCP did not show any toxicity at the therapeutic dose.
In summary, LCP could efficiently evade the RES uptake, and target and penetrate the NSCLC tumor after i.v. injection. Moreover, systemic delivery of pooled siRNA by targeted LCP can provide safe, sequence-specific inhibition of NSCLC tumor growth. Targeted siRNA LCP are efficacious at a relatively low siRNA dose (0.36 mg/kg in total siRNA) and do not require chemical modification of the siRNA for stabilization. Furthermore, LCP do not elicit any detectable immune response or any changes in the host physiology. Future experiments employing pooled siRNA in LCP in combination with small-molecule drugs are likely to further enhance the anti-tumor potency of this system. We therefore conclude that pooled siRNA formulated in targeted LCP has the potential to become a useful tool in NSCLC therapy.
Supplementary Material
Table S1. Primers used for qPCR
Acknowledgments
We thank Srinivas Ramishetti for the synthesis of DSPE-PEG-AA. The work was supported by NIH grants CA149363, CA129835 and CA151652.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 3.Molina JR, Adjei AA, Jett JR. Advances in chemotherapy of non-small cell lung cancer. Chest. 2006;130(4):1211–9. doi: 10.1378/chest.130.4.1211. [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Liu Y. In vivo delivery of RNAi with lipid-based nanoparticles. Annu Rev Biomed Eng. 2011;13:507–30. doi: 10.1146/annurev-bioeng-071910-124709. [DOI] [PubMed] [Google Scholar]
- 5.Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, Molema G, Lu PY, Scaria PV, Woodle MC. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32(19):e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158(1):108–114. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeGeros RZ. Calcium Phosphate-Based Osteoinductive Materials. Chem Rev. 2008;108(11):4742–4753. doi: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- 8.Epple M, Ganesan K, Heumann R, Klesing J, Kovtun A, Neumann S, Sokolova V. Application of calcium phosphate nanoparticles in biomedicine. J Mater Chem. 2010;20(1) [Google Scholar]
- 9.Cheang T-y, Wang S-m, Hu Z-j, Xing Z-H, Chang G-q, Yao C, Liu Y, Zhang H, Xu A-W. Calcium carbonate/CaIP6 nanocomposite particles as gene delivery vehicles for human vascular smooth muscle cells. J Mater Chem. 2010;20(37):8050–8055. [Google Scholar]
- 10.Li J, Chen Y-C, Tseng Y-C, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142(3):416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez M, Roman E, Santos ES, Raez LE. New targets for non-small-cell lung cancer therapy. Expert Rev Anticancer Ther. 2007;7(10):1423–1437. doi: 10.1586/14737140.7.10.1423. [DOI] [PubMed] [Google Scholar]
- 12.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 13.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends Mol Med. 2007;13(1):23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Ponzielli R, Katz S, Barsyte-Lovejoy D, Penn LZ. Cancer therapeutics: Targeting the dark side of Myc. Eur J Cancer. 2005;41(16):2485–2501. doi: 10.1016/j.ejca.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6(8):635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 16.Kaya A, Çiledag A, Gulbay BE, Poyraz BM, Çelik G, Sen E, Savas H, Savas I. The prognostic significance of vascular endothelial growth factor levels in sera of non-small cell lung cancer patients. Respir Med. 2004;98(7):632–636. doi: 10.1016/j.rmed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Shimanuki Y, Takahashi K, Cui R, Hori S, Takahashi F, Miyamoto H, Fukurchi Y. Role of Serum Vascular Endothelial Growth Factor in the Prediction of Angiogenesis and Prognosis for Non-small Cell Lung Cancer. Lung. 2005;183(1):29–42. doi: 10.1007/s00408-004-2521-4. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee R, Tyagi P, Li S, Huang L. Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. Int J Cancer. 2004;112(4):693–700. doi: 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- 19.Inuzuka H, Tseng A, Gao D, Zhai B, Zhang Q, Shaik S, Wan L, Ang XL, Mock C, Yin H, Stommel JM, Gygi S, Lahav G, Asara J, Xiao Z-XJ, Kaelin WG, Jr, Harper JW, Wei W. Phosphorylation by Casein Kinase I Promotes the Turnover of the Mdm2 Oncoprotein via the SCF[beta]-TRCP Ubiquitin Ligase. Cancer Cell. 2010;18(2):147–159. doi: 10.1016/j.ccr.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai M-S, Arnold H, Sun X-X, Sears R, Lu H. Inhibition of c-Myc activity by ribosomal protein L11. EMBO J. 2007;26(14):3332–3345. doi: 10.1038/sj.emboj.7601776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, Tolentino MJ. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–6. [PubMed] [Google Scholar]
- 22.Yang Y, Bai Y, Xie G, Zhang N, Ma YP, Chen LJ, Jiang Y, Zhao X, Wei YQ, Deng HX. Efficient inhibition of non-small-cell lung cancer xenograft by systemic delivery of plasmid-encoding short-hairpin RNA targeting VEGF. Cancer Biother Radiopharm. 2010;25(1):65–73. doi: 10.1089/cbr.2009.0692. [DOI] [PubMed] [Google Scholar]
- 23.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 24.Raben D, Helfrich B, Bunn PA. Targeted therapies for non-mall-cell lung cancer: biology, rationale, and preclinical results from a radiation oncology perspective. Int J Radiat Oncol Biol Phys. 2004;59(2):S27–S38. doi: 10.1016/j.ijrobp.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 25.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Li J, Liu F, Huang L. Systemic Delivery of siRNA via LCP Nanoparticle Efficiently Inhibits Lung Metastasis. Mol Ther. 2012;20(3):609–615. doi: 10.1038/mt.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Ishii A, Nishiyama N, Matsumoto S, Ishii T, Yamasaki Y, Kataoka K. PEGylated Calcium Phosphate Nanocomposites as Smart Environment-Sensitive Carriers for siRNA Delivery. Advanced Materials. 2009;21(34):3520–3525. [Google Scholar]
- 28.Epple M, Ganesan K, Heumann R, Klesing J, Kovtun A, Neumann S, Sokolova V. Application of calcium phosphate nanoparticles in biomedicine. J Mater Chem. 2010;20(1):18–23. [Google Scholar]
- 29.Zhang M, Kataoka K. Nano-structured composites based on calcium phosphate for cellular delivery of therapeutic and diagnostic agents. Nano Today. 2009;4(6):508–517. [Google Scholar]
- 30.Kakizawa Y, Furukawa S, Kataoka K. Block copolymer-coated calcium phosphate nanoparticles sensing intracellular environment for oligodeoxynucleotide and siRNA delivery. Journal of Controlled Release. 2004;97(2):345–356. doi: 10.1016/j.jconrel.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 31.Kakizawa Y, Furukawa S, Ishii A, Kataoka K. Organic-inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer. Journal of Controlled Release. 2006;111(3):368–370. doi: 10.1016/j.jconrel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145(3):178–81. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabaković A, Kester M, Adair JH. Calcium phosphate-based composite nanoparticles in bioimaging and therapeutic delivery applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(1):96–112. doi: 10.1002/wnan.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used for qPCR