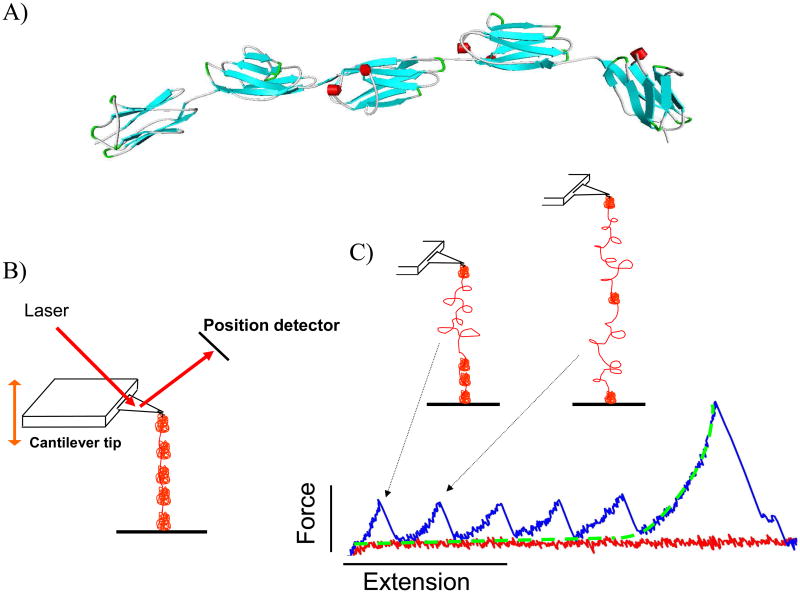
Figure 4.
A) A ribbon schematic of five serially-linked Ig domains. β-strands are colored blue and alpha helices are red. The atomic coordinates were downloaded from the Worldwide Protein Data Bank, PDB ID: 3B43 (Von Castelmur et al. 2008). Each folded Ig domain is 4-5 nm in diameter and connected by short linker sequences. B) Simplified AFM schematic. The cantilever tip probes a protein-coated surface until a molecule is tethered. The molecule is then stretched and the force that develops is determined by measuring the deflection of the laser off the cantilever. C) A tethered polyprotein is stretched to induce domain unfolding. Large structural transitions, such as complete unfolding of an Ig domain, result in a large contour length increase, a release of tension in the molecule, and a sharp unfolding force peak. In the example shown, the molecule is still attached after all five domains unfold; the last force peak is due to stretching the completely unfolded peptide that behaves as an entropic spring and is well-described by the WLC equation (fitted dashed line).