Figure 5.
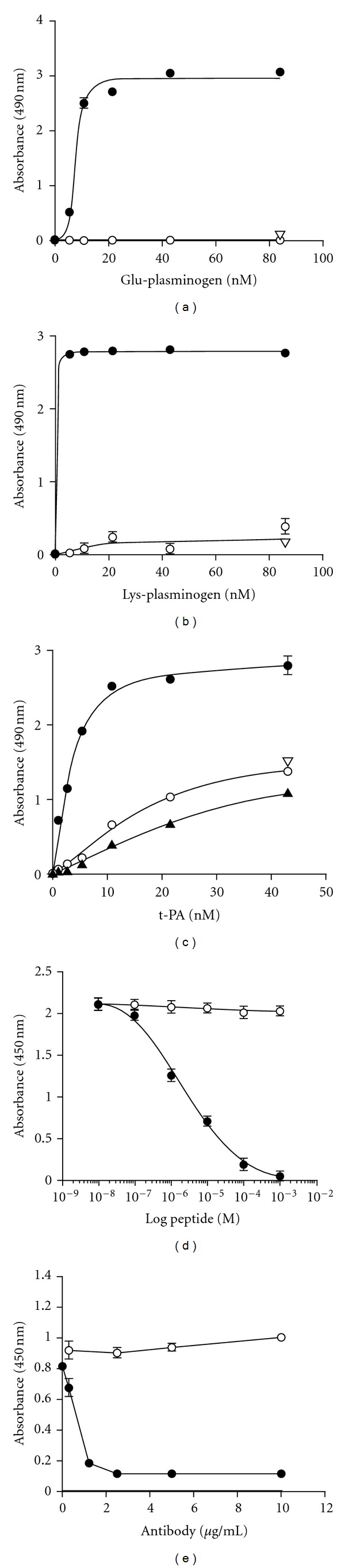
Plasminogen binds to the C-terminal peptide of Plg-RKT. The peptide, CEQSKLFSDK, corresponding to the amino terminus of Plg-RKT was coupled to BSA and coated onto wells of microtiter plates. Either Glu-plasminogen (a) or Lys-plasminogen (b) or t-PA (c) was then incubated with the wells, followed by antiplasminogen mAb [19] (a, b) or anti-t-PA polyclonal mAb (c) and detection with HRP-conjugated goat anti-mouse IgG (closed circles) as described in Materials and Methods. The binding was specific because it was blocked in the presence of 0.2 M EACA (open triangles), consistent with the ability of EACA to inhibit plasminogen binding to differentiated Hoxa9-ER4 cells. In additional controls, nonspecific binding to either BSA (closed triangles) or to the reverse peptide (open circles) was <10% of binding to CEQSKLFSDK. (At high input concentrations of t-PA nonspecific binding increased but was <10% of binding to CEQSKLFSDK at the concentration required for 50% saturation (3.2 nM). In controls for the detection method, O.D.490 values obtained using an isotype control antibody or in the absence of added plasminogen or t-PA were <5% of the values for plasminogen or t-PA binding to immobilized CEQSKLFSDK. Panels d and e: biotinylated-Glu-plasminogen (25 nM) was incubated with immobilized CEQSKLFSDK in the presence of increasing concentrations of (d) the C-terminal peptide, CEQSKLFSDK (closed circles), or a mutated C-terminal peptide with K147 substituted with alanine, CEQSKLFSDA (open circles) or (e) anti-Plg-RKT mAb35B10 (closed circles) or isotype control (open circles). Biotinylated Glu-plasminogen binding was detected with HRP-streptavidin and was 96% inhibited in the presence of 0.2 M EACA (not shown). Data are as mean ± SEM, n = 3, for each determination. This research was originally published in [18].
