Figure 5.
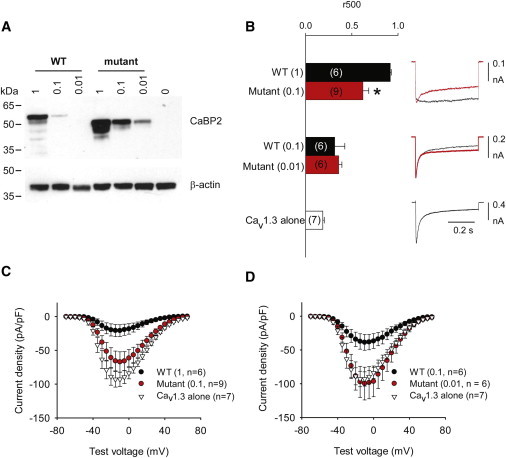
c.637+1G>T Mutation Impairs CaBP2 Regulation of Cav1.3 Ca2+ Channels
(A) The MT CaBP2 shows greater protein levels in transfected HEK 293T cells. Lysates of cells cotransfected with Cav1.3 subunits and WT or MT CaBP2 were subject to immunoblotting with CaBP2 antibodies (top panel). WT and MT CaBP2 were cotransfected at different molar ratios as indicated. The lower panel was probed with β-actin antibodies for confirming equal loading between groups.
(B) The MT CaBP2 shows weaker regulation of CDI. HEK 293T cells were cotransfected as in (A). Ca2+ currents were evoked by 0.5 s pulses from −90 mV to −10 mV. Inactivation was measured as r500, which was the current amplitude at the end of the pulse normalized to the peak current amplitude. So that different protein levels (also in C) could be accounted for, comparisons to WT were made with cells transfected with cDNA encoding MT CaBP2 at levels ten times lower than those of WT CaBP2. Parentheses indicate numbers of cells. ∗p < 0.05. Error bars represent the SEM.
(C and D) The MT CaBP2 shows a weaker ability to suppress Cav1.3 current density than does the WT CaBP2. Ca2+ currents were evoked by 50 ms pulses to variable voltages from −90 mV. Current amplitude was normalized to cell capacitance and plotted against test voltage. Error bars represent the SEM.
