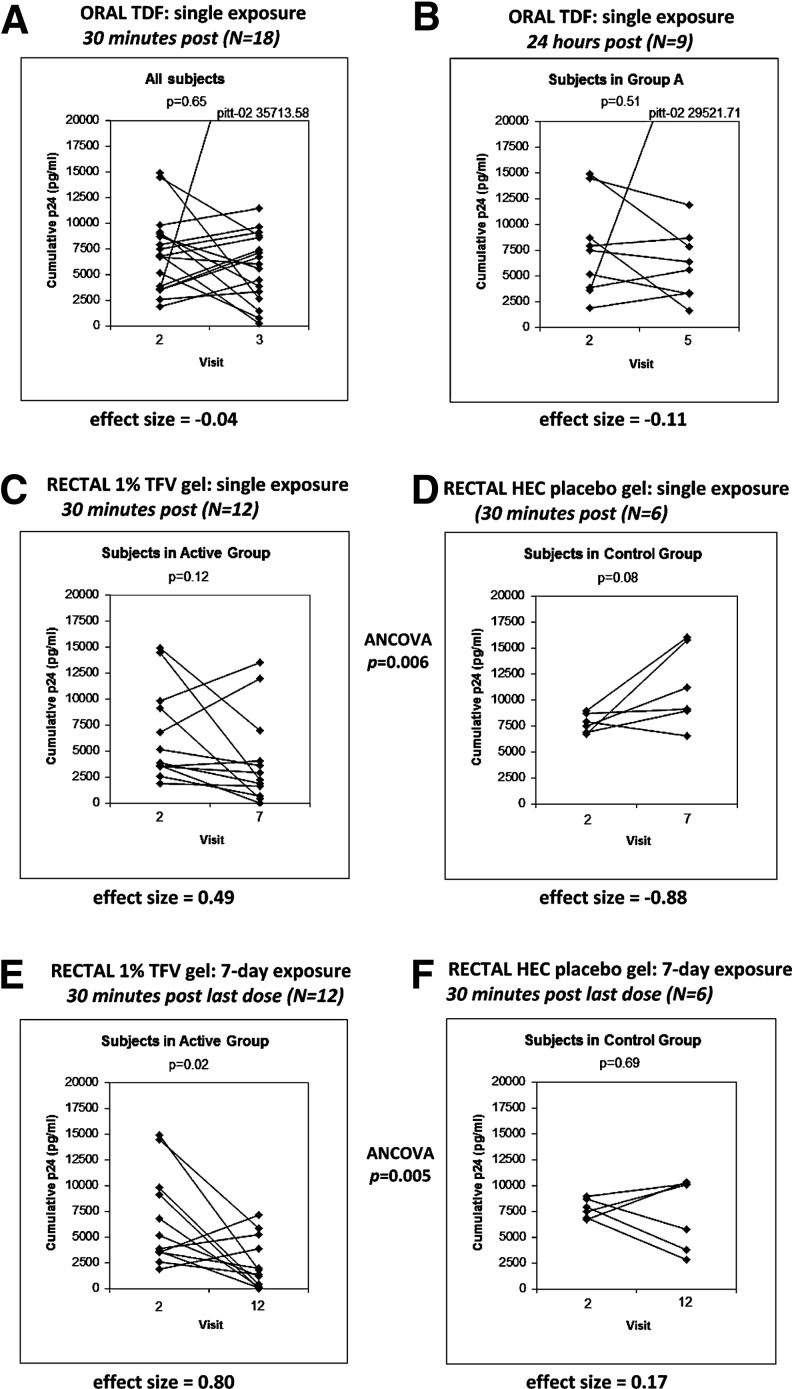
FIG. 4.
Changes in ex vivo infectibility by HIVBaL 104 TCID50 of rectal biopsies following TVF exposure. Graphs document the changes in cumulative p24 from ex vivo tissue infectibility at V2 (all baseline; no product exposure) compared to the same subject's tissue samples collected/exposed ex vivo following product exposure. (A and B) Show changes following single-oral exposure. As there was no placebo group during the oral-exposure stage, results from 30 minutes (A) and 24 hours (B) post-exposure are shown to demonstrate absence of delayed suppressive effects due to a systemically-delivered oral drug (B). (C and D) Show results at baseline (each graph: left side) and 30 minutes after (each graph: right side) single topical exposure to TFV gel (C) compared to HEC placebo (D). (E and F) Show the same arrangement 30 minutes following 7-days topical exposure. The points for the same individual are linked by a solid line. The p-value of a paired test (Wilcoxon for A,B; t-test for C–F) is displayed within each plot; effect sizes are displayed under each plot and ANCOVAs, showing differences between TFV-exposed and placebo groups, after adjusting for baseline measurements, are placed between respective graphs.