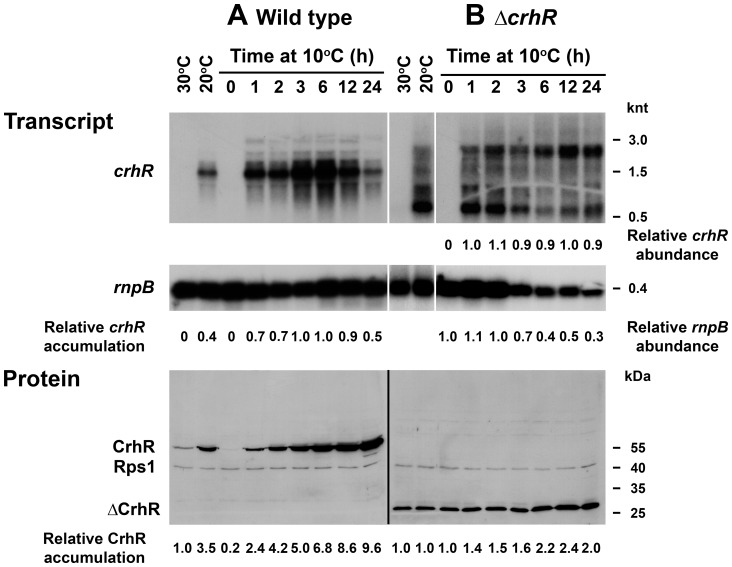
Figure 7. Time course of crhR expression at 10°C.
Wild type (A) and ΔcrhR (B) Synechocystis were grown to mid-log phase at 30°C and transferred to 10°C for the indicated times. Samples were harvested for RNA and soluble protein extraction at the indicated time points. Cells grown at 30°C and 20°C are included as references. (A) The relative crhR accumulation in comparison with the abundance in illuminated, wild type cells grown at 30°C corrected for rnpB levels (set to 1.0), is given below each lane for wild type cells. For ΔcrhR cells, decreasing rnpB accumulation necessitated calculation of the relative abundance of crhR and rnpB independently. Transcript levels at 1 h of exposure to 10°C and 0 time were set to 1.0 for crhR and rnpB, respectively. Quantification of the 2.3 and 0.75 knt transcripts is shown in Table S1. (B) Western blots were simultaneously probed with antibodies against CrhR and E. coli Rps1 that served as a control for protein loading. The relative fold change in CrhR and ΔCrhR accumulation, corrected for Rps1 levels, as described in Methods S1, is given below each lane. Protein samples were resolved on separate gels, hence normalization was performed independently for the wild type and mutant, with abundance observed in wild type and mutant at 30°C set to 1.0.