Abstract
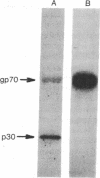
Antibodies in human sera from healthy individuals were shown to be reactive with highly purified 70,000-dalton envelope glycoprotein (gp70) of the simian sarcoma virus-simian sarcoma-associated virus (SSV-SSAV) complex in radioimmunoprecipitation assays under certain conditions. The specificity of the reaction was analyzed in absorption tests with normal human serum proteins, assays of viral gp70 antigenicity after exposure to exo- and endoglycosidases or trypsin, and carbohydrate hapten inhibition studies. On the basis of the results obtained in these experiments we have concluded that immune recognition of SSV-SSAV gp70 can be mediated by naturally occurring heterophil antibodies in human sera that are reactive by virtue of binding to the carbohydrate moiety of the viral gp70 molecules. The results are consistent with the idea that the antibodies in question are elicited as a result of exposure to many natural substances possessing widely crossreacting antigens and are not a result of widespread infection of man with replication-competent oncoviruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Walling M. J., Bushar G. S., Liu M., Hsu K. C. Natural antibodies in sera from healthy humans to antigens on surfaces of type C RNA viruses and cells from primates. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2491–2495. doi: 10.1073/pnas.73.7.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Bolognesi D., Aaronson S. A. Humans have antibodies capable of recognizing oncoviral glycoproteins: demonstration that these antibodies are formed in response to cellular modification of glycoproteins rather than as consequence of exposure to virus. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1617–1621. doi: 10.1073/pnas.77.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman H. P., Kim N., White M., Gilden R. V. Failure to detect, in human sera, antibodies cross-reactive with group-specific antigens of murine leukemia virus. J Natl Cancer Inst. 1974 May;52(5):1409–1413. doi: 10.1093/jnci/52.5.1409. [DOI] [PubMed] [Google Scholar]
- Charman H. P., Kim N., White M., Marquardt H., Gilden R. V., Kawakami T. Natural and experimentally induced antibodies to defined mammalian type-C virus proteins in primates. J Natl Cancer Inst. 1975 Dec;55(6):1419–1424. doi: 10.1093/jnci/55.6.1419. [DOI] [PubMed] [Google Scholar]
- Charman H. P., Rahman R., White M. H., Kim N., Gilden R. V. Radioimmunoassay for the major structural protein of Mason-Pfizer monkey virus: Attempts to detect the presence of antigen or antibody in humans. Int J Cancer. 1977 Apr 15;19(4):498–504. doi: 10.1002/ijc.2910190410. [DOI] [PubMed] [Google Scholar]
- Ebbesen P., Due C., Hesse J., Kurth R., Noble G. R., Gallagher R., Voller A., Jensen G. Elevated titer of antibodies to Simian sarcoma virus envelope antigen (gp70) and normal response to influenza virus in untreated Danish Hodgkin's patients. Int J Cancer. 1979 Jul 15;24(1):1–5. doi: 10.1002/ijc.2910240102. [DOI] [PubMed] [Google Scholar]
- Famulari N. G., Jelalian K. Cell surface expression of the env gene polyprotein of dual-tropic mink cell focus-forming murine leukemia virus. J Virol. 1979 Jun;30(3):720–728. doi: 10.1128/jvi.30.3.720-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. D., Jr Immunodiffusion studies of feline leukemia and sarcoma. J Am Vet Med Assoc. 1971 Mar 15;158(6 Suppl):1060+–1060+. [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Antigenic determinants of the 70,000 molecular weight glycoprotein of woolly monkey type C RNA virus. J Immunol. 1975 Oct;115(4):922–927. [PubMed] [Google Scholar]
- Hirsch M. S., Kelly A. P., Chapin D. S., Fuller T. C., Black P. H., Kurth R. Immunity to antigens associated with primate C-type oncoviruses in pregnant women. Science. 1978 Mar 24;199(4335):1337–1340. doi: 10.1126/science.204010. [DOI] [PubMed] [Google Scholar]
- Hirshaut Y., Pei D. T., Marcove R. C., Mukherji B., Spielvogel A. R., Essner E. Seroepidemiology of human sarcoma antigen (S1). N Engl J Med. 1974 Nov 21;291(21):1103–1107. doi: 10.1056/NEJM197411212912103. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Gallo R. C. Characterization of Rauscher murine leukemia virus envelope glycoprotein receptor in membranes from murine fibroblasts. J Virol. 1978 Dec;28(3):686–696. doi: 10.1128/jvi.28.3.686-696.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Sefton B., Burke D. Sindbis virus glycoproteins: effect of the host cell on the oligosaccharides. J Virol. 1975 Sep;16(3):613–620. doi: 10.1128/jvi.16.3.613-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. C., Basak S., Compans R. W. Glycopeptides of murine leukemia viruses. I. Comparison of two ecotropic viruses. J Virol. 1979 Jul;31(1):1–7. doi: 10.1128/jvi.31.1.1-7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower J. M., Aaronson S. A. Seroepidemiologic assessment of feline leukaemia virus infection risk for man. Nature. 1978 Jun 8;273(5662):463–464. doi: 10.1038/273463a0. [DOI] [PubMed] [Google Scholar]
- Krakower J. M., Tronick S. R., Gallagher R. E., Gallo R. C., Aaronson S. A. Antigenic characterization of a new gibbon ape leukemia virus isolate: seroepidemiologic assessment of an outbreak of gibbon leukemia. Int J Cancer. 1978 Dec;22(6):715–720. doi: 10.1002/ijc.2910220613. [DOI] [PubMed] [Google Scholar]
- Kurth R., Mikschy U. Human antibodies reactive with purified envelope antigens of primate type C tumor viruses. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5692–5696. doi: 10.1073/pnas.75.11.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth R., Teich N. M., Weiss R., Oliver R. T. Natural human antibodies reactive with primate type-C viral antigens. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1237–1241. doi: 10.1073/pnas.74.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDUC E. H., TANAKA N. A study of the cellular distribution of Forssman antigen in various species. J Immunol. 1956 Sep;77(3):198–212. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Louie S., Curtis J. E., Till J. E., McCulloch E. A. Antibodies in human sera to oncorna virus-like proteins from normal or leukemia marrow cell cultures. J Exp Med. 1976 Nov 2;144(5):1243–1253. doi: 10.1084/jem.144.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Ledda C. A., Freiman H. J., Choppin P. W. Biological properties of the VSV glycoprotein. II. Effects of the host cell and of the glycoprotein carbohydrate composition on hemagglutination. Virology. 1978 Jan;84(1):183–188. doi: 10.1016/0042-6822(78)90230-1. [DOI] [PubMed] [Google Scholar]
- Moennig V., Frank H., Hunsmann G., Schneider I., Schafer W. Properties of mouse leukemia viruses. VII. The major viral glycoprotein of friend leukemia virus. Isolation and physicochemical properties. Virology. 1974 Sep;61(1):100–111. doi: 10.1016/0042-6822(74)90245-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Glycopeptide components of influenza viral glycoproteins. Virology. 1978 May 15;86(2):432–442. doi: 10.1016/0042-6822(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Kirsten W. H. Inhibition of reverse transcriptases of primate type C viruses by 7S immunoglobulin from patients with leukaemia. Nature. 1976 Mar 4;260(5546):64–67. doi: 10.1038/260064a0. [DOI] [PubMed] [Google Scholar]
- Robertson M. A., Etchison J. R., Robertson J. S., Summers D. F., Stanley P. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistnat CHO cells. Cell. 1978 Mar;13(3):515–526. doi: 10.1016/0092-8674(78)90325-2. [DOI] [PubMed] [Google Scholar]
- Rogentine G. N., Jr, Plocinik B. A. Carbohydrate inhibition studies of the naturally occurring human antibody to neuraminidase-treated human lymphocytes. J Immunol. 1974 Sep;113(3):848–858. [PubMed] [Google Scholar]
- Sarma P. S., Sharar A., Walters V., Gardner M. A survey of cats and humans for prevalence of feline leukemia-sarcoma virus neutralizing serum antibodies. Proc Soc Exp Biol Med. 1974 Feb;145(2):560–564. doi: 10.3181/00379727-145-37851. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Gottlieb C., Feil P., Gelb N., Kornfeld S. Growth of enveloped RNA viruses in a line of chinese hamster ovary cells with deficient N-acetylglucosaminyltransferase activity. J Virol. 1975 Jan;17(1):239–246. doi: 10.1128/jvi.17.1.239-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Mosquito cells infected with vesicular stomatitis virus yield unsialylated virions of low infectivity. J Virol. 1975 Apr;15(4):1029–1032. doi: 10.1128/jvi.15.4.1029-1032.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma. III. Recognition of autoantibodies with unusual characteristics. J Exp Med. 1977 Mar 1;145(3):784–789. doi: 10.1084/jem.145.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Fox M. Characterization of a fetal calf serum-derived molecule reactive with human natural antibodies: its occurrence in tissue culture-grown type C RNA viruses. J Immunol. 1978 Feb;120(2):646–651. [PubMed] [Google Scholar]
- Snyder H. W., Jr, Pincus T., Fleissner E. Specificities of human immunoglobulins reactive with antigens in preparations of several mammalian type-C viruses. Virology. 1976 Nov;75(1):60–73. doi: 10.1016/0042-6822(76)90007-6. [DOI] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Stockert E., Fleissner E. Characterization of molecular species carrying gross cell surface antigen. J Virol. 1977 Aug;23(2):302–314. doi: 10.1128/jvi.23.2.302-314.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Search for antigens and antibodies crossreactive with type C viruses of the woolly monkeys and gibbon ape in animal models and in humans. Proc Natl Acad Sci U S A. 1976 May;73(5):1725–1729. doi: 10.1073/pnas.73.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]