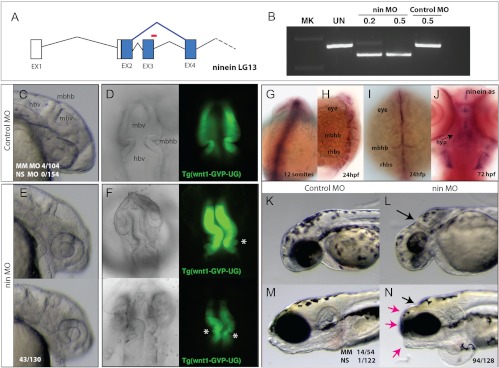
Fig. 4.
Specific developmental role of ninein in the formation of the brain and shape of the skull. A, Splice blocking morpholino oligonucleotide (red, nin MO) was designed against ninein exon 3 and is predicted to cause altered splicing (blue), resulting in a frame shift in the coding sequence of the resulting message. B, RT-PCR of ninein message after injection of control or nin MO 24 hpf. C and E, Morphology of forming brain of the zebrafish at 24 hpf embryos injected with control or nin MO. D and F, Dorsal views of the forming anterior neural folds of 24-hpf embryos in wnt1:GFP transgenic fish [Tg(wnt1-GVP-UG)] highlight early neuroectodermal structures and initiation of differentiation in larvae injected with control (D) or nin MO (F; asterisk, aberrant folds found rostral to the MBHB). G–J, Whole-mount in situ analysis of ninein expression. G, Expression of ninein at 12 somite limited to neuroectodermal structures of the rostral and caudal neural tube. H and I, Expression of ninein in 24-hpf embryos resolves to mark anterior neural folds as well as the forming rhobomeres. J, Ventral view of 72-hpf zebrafish showing specific expression of ninein in the forming hypothalamus. K and L, Knockdown of ninein results in anterior deficiencies of the skull showing reduced eye and skull growth, specifically in the area of the MBHB (arrow). M and N, Morphology of 5-dpf larvae of control (M) and nin MO-treated groups showed specific shortening of the skull (red arrows) coincident with smaller eyes, otoliths, and anterior clefts at the site of the MBHB. hbv, Hindbrain ventricle; hyp, hypothalamus; mbhb, midbrain hindbrain isthmus fold; mbv, midbrain ventricle; rhbs, rhombomeres.