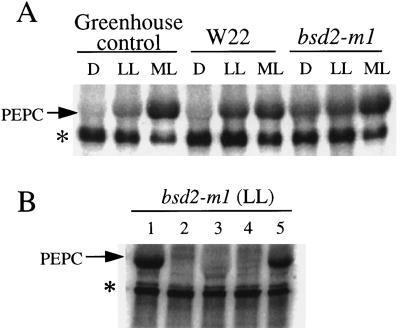
Figure 3.
In vitro assays of PEPC kinase activity. A, Determination of PEPC kinase activity in desalted crude extracts from greenhouse-grown control maize and chamber-grown W22 and bsd2-m1 leaves harvested after 8 h of darkness (D), after 2 h of low light (LL), and after a further 2 h of illumination at a more moderate light (ML) intensity. Ten micrograms of total soluble protein was assayed for PEPC kinase activity in the presence of [γ-32P]ATP/Mg (8 μCi), 0.5 mm EGTA, 0.1 μm MC-LR, and 10 μg of purified dephospho (dark form) wild-type maize PEPC. The arrow denotes the approximately 110-kD PEPC polypeptide on this representative phosphorimage. An unknown contaminating phosphoprotein may also be seen (denoted by the asterisk). This phosphoprotein was neither detected by Coomassie blue staining of the gel before phosphorimaging (data not shown) nor derived from the exogenous PEPC substrate (see lane 2 in B). B, Substrate and phosphorylation-site specificity of bsd2-m1 kinase activity. Low-light (LL)-illuminated bsd2-m1 leaves were extracted and assayed for PEPC kinase activity (10 μg of desalted soluble protein) as described for A, with or without 10 μg of purified exogenous recombinant wild-type (Ser-8) or phosphorylation-site mutant sorghum C4 PEPC in the in vitro phosphorylation medium. Lane 1, Plus Ser-8 PEPC; lane 2, minus exogenous PEPC (control); lane 3, plus S8D PEPC; lane 4, plus S8Y PEPC; and lane 5, plus S8T PEPC. The arrow denotes the position of the PEPC polypeptide on this representative phosphorimage.