Fig. 2.
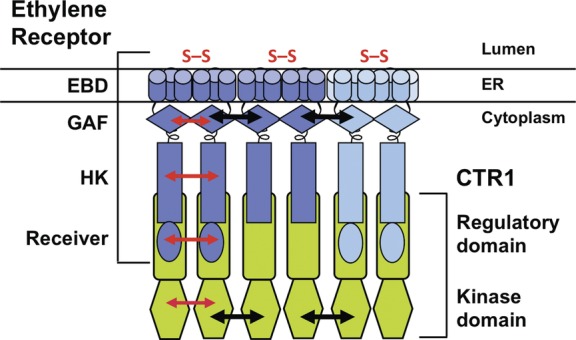
Model of an active heteromeric ethylene receptor–CTR1 complex at the ER membrane. The ethylene receptors are tethered to the ER membrane (Chen et al. 2002, 2007; Dong et al. 2008; Grefen et al. 2008) by the N-terminal ethylene-binding domain (EBD). Representative ethylene receptors of subfamily I (in dark blue) and subfamily II (in light blue) are homodimers (Schaller and Bleecker 1995; Gao et al. 2008), each stabilized by a pair of intermolecular N-terminal disulfide bonds (S–S) in the lumen (Schaller et al. 1995), as well as likely non-covalent interactions (red arrows, shown only on the leftmost homodimer) between the two-component histidine kinase domains (HK), receiver domains and GAF domains. The receptor homodimers form a higher-order complex with neighbouring receptor dimers, mediated in part by the GAF domain (black arrows) (Schaller et al. 1995; Hall et al. 2000; Gao et al. 2008; Grefen et al. 2008). The N-terminal regulatory domain of the CTR1 protein kinase (green) physically associates with the HK and receiver domains of the receptors (Clark et al. 1998; Cancel and Larsen 2002; Gao et al. 2003; Zhong et al. 2008). We speculate that each receptor HK domain associates with one CTR1 molecule. Based on crystal structures, the ETR1 receiver domain (Müller-Dieckmann et al. 1999) and the CTR1 kinase domain (Mayerhofer et al. 2012) are each dimers (red arrows). The CTR1 kinase domain is believed to be active when dimerized (Mayerhofer et al. 2012). Moreover, oligomerization of the CTR1 kinase domain dimers (black arrows) may help to bring the ethylene receptors together, reinforcing the receptor complex (Mayerhofer et al. 2012). The receptors have also been found in high-molecular-mass complexes containing unidentified proteins (Chen et al. 2010) (not shown). The higher-order ethylene receptor complexes may serve to amplify the signal.
