Fig 3.
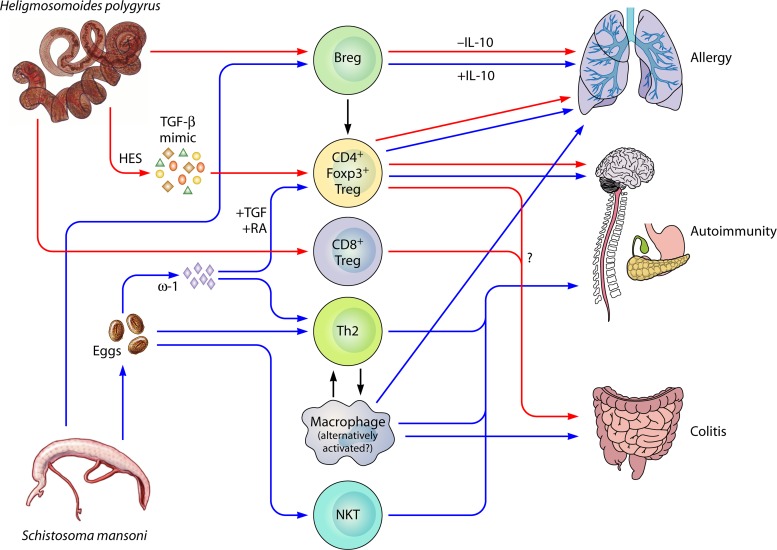
Suppressive mechanisms of Heligmosomoides polygyrus and Schistosoma mansoni on immunopathologies. Of all the helminths that infect mice, the effects of H. polygyrus (red arrows) and S. mansoni (blue arrows) on immunopathology models have been best characterized. Both parasites effectively suppress immune responses and pathology in models of allergy, autoimmunity, and colitis. CD4+ Foxp3+ (Forkhead box p3) Tregs (regulatory T cells) are induced by both H. polygyrus and S. mansoni through secretory molecules affecting the TGF-β (transforming growth factor β) and/or retinoic acid pathways, and these Tregs can suppress all immunopathologies. Suppressive Bregs (regulatory B cells) are also induced by both parasites, and functional suppression has been shown in allergy and autoimmunity through B cell IL-10 production (S. mansoni) or an IL-10-independent mechanism (H. polygyrus). H. polygyrus has also been shown to induce a regulatory population of CD8+ T cells in the gut, which may, along with CD4+ Foxp3+ Tregs, be involved in the suppression of colitis in an IL-10-independent, TGF-βR-dependent manner. S. mansoni eggs and their products (especially the RNase ω-1) are powerful Th2 (T helper type 2) inducers, and skewing toward a Th2 response is protective in some models of autoimmunity. Th2 cytokines also induce the alternative activation of macrophages, which are functionally suppressive. Macrophages induced by S. mansoni infections (whether alternatively activated or not) are suppressive in all models of immunopathology studied. Finally, S. mansoni eggs induce the expansion of NKT (natural killer T) cells, which are deficient in the diabetic NOD mouse, and protect against disease.