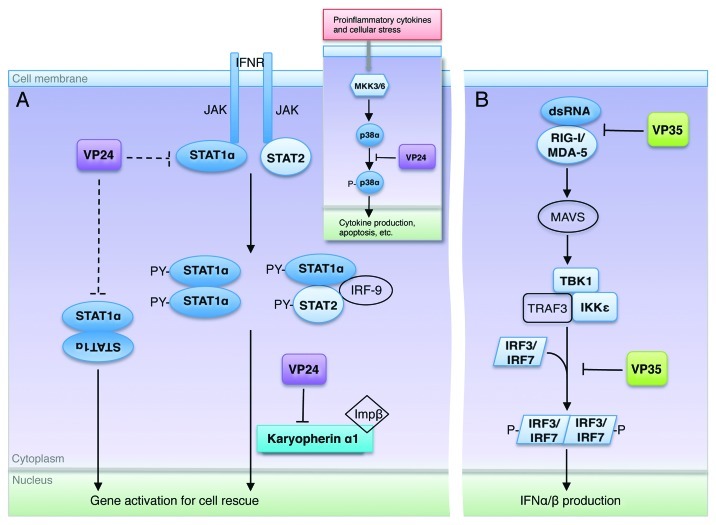
Figure 3. Excerpts from the ebolavirus operation plan. (A) Upon interferon activation, STAT1 becomes phosphorylated by JAK and forms either an ISG3 complex (STAT1-STAT2-IRF9) or a homodimer. The STAT1 complex is then translocated to the nucleus by the carrier protein karyopherin α1. VP24 targets karyopherin α1 to sequester STAT1 in the cytoplasm. VP24 may also be able to target STAT1 directly (interactions shown here in dashed lines), although the downstream result of this binding is not yet known. Inset shows that VP24 also interferes in the MKK3/6-p38 mitogen-activated protein (MAP) kinase pathway,11 by blocking IFNβ stimulated phosphorylation of p38-α. (B) The presence of double-stranded RNA is detected by the sensors RIG-I and MDA-5 that then trigger signaling of mitochondrial antiviral signaling proteins (MAVS), also known as virus-induced signaling adapter (VISA), IPS-1 and Cardif to activate Tank binding kinase-1 (TBK-1) and I-Kappa-B kinase epsilon (IKKε). With the aid of TNF receptor-associated factor 3 (TRAF3), activated TBK-1 and IKKε then phosphorylate interferon regulatory factor (IRF) 3 and IRF7. Upon phosphorylation, IRF3 and IRF7 homodimerize, translocate to the nucleus, and subsequently initiate production of type I IFNs. VP35 binds and masks dsRNA to prevent this signaling and downstream expression of IFNs. In addition, VP35 can also inhibit the phosphorylation of IRF321 by interacting with the kinase domain of IKKε22. In this way, both VP35 and VP24 may interfere at multiple steps in their respective pathways.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.