Abstract
The transcription factor hypoxia-inducible factor 1 (HIF-1) has recently emerged to be a crucial regulator of the immune response following pathogen perception, including the response to the important human pathogen Pseudomonas aeruginosa. However, as mechanisms involved in HIF-1 activation by bacterial pathogens are not fully characterized, understanding how bacteria and bacterial compounds impact on HIF-1α stabilization remains a major challenge. In this context, we have focused on the effect of secreted factors of P. aeruginosa on HIF-1 regulation. Surprisingly, we found that P. aeruginosa cell-free supernatant significantly repressed HIF-1α protein levels. Further characterization revealed that HIF-1α downregulation was dependent on a subset of key secreted factors involved in P. aeruginosa pathogenesis, the 2-alkyl-4-quinolone (AQ) quorum sensing (QS) signaling molecules, and in particular the pseudomonas quinolone signal (PQS). Under hypoxic conditions, the AQ-dependent downregulation of HIF-1α was linked to the suppressed induction of the important HIF-1 target gene hexokinase II. Furthermore, we demonstrated that AQ molecules directly target HIF-1α protein degradation through the 26S-proteasome proteolytic pathway but independently of the prolyl hydroxylase domain (PHD). In conclusion, this is the first report showing that bacterial molecules can repress HIF-1α protein levels. Manipulation of HIF-1 signaling by P. aeruginosa AQs could have major consequences for the host response to infection and may facilitate the infective properties of this pathogen.
INTRODUCTION
The ability of the human host to respond effectively to microbial infection requires the rapid implementation of molecular and cellular responses via activation of a battery of transcription factors, of which NF-κB is the best known (9). In recent years, the hypoxia-inducible factor-1 (HIF-1) transcription factor has emerged as a crucial component of the host defense in response to inflammation (6) and infection (13, 34, 59). HIF-1, described as the master regulator of the hypoxic response (53), is composed of two protein subunits, HIF-1α and HIF-1β. However, HIF-1 transcriptional activity is principally dependent on HIF-1α protein stabilization (53). Whereas the HIF-1β subunit is mainly constitutively expressed and stable within the cells, HIF-1α protein expression is strongly regulated at a posttranslational level. The prolyl hydroxylase domain (PHD) enzymes are one of the main regulators of HIF-1α protein expression, through an oxygen-dependent hydroxylation mechanism (21, 46). Hydroxylated HIF-1α proteins are subsequently recognized and bound by the von Hippel-Lindau (pVHL) ubiquitin E3 ligase complex, labeling HIF-1α with ubiquitin protein, in what is the final step of HIF-1α degradation by the 26S proteasome machinery (21, 46). Hypoxia, by inhibiting PHD activity, consequently prevents HIF-1α degradation, leading to activation of HIF-1. Target genes of HIF-1 regulate several biological processes, including cell survival, angiogenesis, and energy metabolism, that allow the cell, tissue, and organism to adapt to cellular stress caused by oxygen deficiency (47).
Recent research has suggested that activation of HIF-1 also occurs in response to infections with human pathogens (55). To date, a number of Enterobacteriaceae species (18), including Escherichia coli (3), Chlamydia (44, 49), Pseudomonas aeruginosa (26, 40, 48), and group A Streptococcus (39, 40), have all been shown to stabilize HIF-1α in both immune and epithelial cells. Stabilization of HIF-1α and subsequent HIF-1 activation promote the release of antimicrobial peptides such as cathelicidins and granule proteases and stimulate the production of nitric oxide and tumor necrosis factor alpha (TNF-α) (34, 40). However, whereas the role of HIF-1 in response to infection has been clearly established, molecular mechanisms underpinning stabilization of HIF-1α by bacterial pathogens are not fully characterized. General mechanisms linked to infection itself, such as the hypoxic environment created by bacterial oxygen consumption, have been proposed to be in part responsible for HIF-1 activation upon infection (34). Moreover, microbial factors such as chemically synthesized lipopolysaccharide (LPS) from E. coli (15) and purified siderophores from Enterobacteriaceae (18) have also been shown to be involved in the stabilization of HIF-1α.
However, in the case of P. aeruginosa, the predominant pathogen associated with respiratory disease and morbidity and mortality in cystic fibrosis (CF) patients (36), the exact mechanism(s) by which P. aeruginosa causes activation of the HIF-1 pathway is still not known. P. aeruginosa is known to produce a range of extracellular virulence factors, such as proteases, exotoxins, and siderophores, which are crucial for the initial colonization and persistence of the infection (4). Many of the virulence factors secreted by P. aeruginosa have been shown to be regulated by a cell density-dependent gene regulatory signaling mechanism known as quorum sensing (QS). QS systems are activated upon accumulation of signal molecules and control the production of virulence factors above a threshold or quorum. Since the secretion of these extracellular molecules is more beneficial at higher cell densities (7), QS systems provide an exquisite level of control over the expression of key cellular traits linked to virulence and pathogenesis. In addition to the acyl homoserine lactone (AHL) QS class, the 2-alkyl-4-quinolone (AQ) family plays a key role in the P. aeruginosa QS network (12, 20). Among the 56 AQs produced by P. aeruginosa (27), pseudomonas quinolone signal (PQS) and its immediate precursor 2-heptyl-4-quinolone (HHQ) are the best characterized and have been detected in sputum of CF patients (5, 16, 29), indicating that they are secreted into the host environment.
Therefore, in light of the recent reports describing the significant impact of P. aeruginosa secreted factors such as pyocyanin (17) and QS signal molecules, including both AHLs (32) and AQs (24), on gene expression and the immune response in eukaryotic cells, we decided to investigate the effect of P. aeruginosa extracellular factors on HIF-1. As to date, bacteria and bacterial compounds have been shown to lead to HIF-1α protein accumulation (3, 15, 18, 25, 26, 39, 40, 44, 49, 55), it was therefore unexpected to find that P. aeruginosa AQ signaling molecules suppressed HIF-1α protein levels. Destabilization occurred through degradation by the 26S proteasome machinery and resulted in modulation of the HIF-1 target gene HKII. To our knowledge this is the first report of a bacterial factor known to suppress HIF-1α stabilization.
MATERIALS AND METHODS
Bacterial strains and reagents.
Wild-type (wt) PA14 and the transposon insertion mutants PA14_MrT7::pqsA (PA0996) (mutant ID 23621), PA14_MrT7::pqsH (PA2587) (mutant ID 47950), and PA14_MrT7::pqsL (PA4190) (mutant ID 45060) were obtained from the P. aeruginosa transposon mutant library (28), and insertion was verified by PCR. P. aeruginosa PAO1 wt was originally obtained from B. Iglewski. All the strains were cultivated routinely in LB medium at 37°C under agitation (150 rpm), unless stated otherwise. All reagents were obtained from Sigma-Aldrich, unless stated otherwise.
Cell culture.
IB3-1 (ATCC CRL-2777) is a bronchial epithelial cell line derived from a CF patient with CFTR ΔF508/W1282X alleles purchased from the American Type Culture Collection (ATCC, LGC Standards). S9 cells (ATCC CRL-2778) are IB3-1 cells corrected for CFTR expression by transfection with wild-type adeno-associated viral CFTR (AAVCFTR). CFTE29o− is a tracheo-bronchial epithelial cell line derived from a CF patient with CFTR ΔF508/ΔF508 alleles obtained as a gift from D. C. Gruenert. A549 (ATCC CCL-185) is a lung adenocarcinoma epithelial cell line derived for a patient with a lung carcinoma. IB3-1 and S9 cells were cultured using LHC-8 medium (Invitrogen) as previously described (26). CFTE and A549 cells were cultured using minimal essential medium (MEM) M7278 supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere and used up to passage twenty. Hypoxic conditions (1% O2) were created using a hypoxia chamber (Coy Scientific) by injection of nitrogen gas. For all experiments, cells were used when the confluence reached 80% and all treatments were performed for 16 h.
P. aeruginosa cell-free culture supernatants and 2-alkyl-4-quinolone (AQ) extract preparation.
Bacterial strains were cultured with a starting optical density (OD) of 0.05 at 600 nm for 7 h in LHC-8 medium (Invitrogen). The OD was adjusted to 1.8, corresponding to the late exponential phase of growth, and supernatants were collected by centrifugation (4,000 × g for 10 min) and filtered twice using 0.22-μm pores. Similar culture conditions were used for the preparation of the AQ extracts except that strains were grown in LB and centrifugation was performed at 10,000 × g. Extraction of AQs by acidified ethyl acetate was performed as described by Fletcher et al. (14). Extracts were dried and concentrated 200× in methanol. The quality of the P. aeruginosa extracts were verified by thin-layer chromatography (TLC) as previously described (14). All extracts were diluted 1/5, 1/15, 1/25, and 1/50 in cell culture medium, which is equivalent to multiplicities of infection (MOIs) of 500, 166, 100, and 50 to 1 eukaryotic cell, respectively.
Western blot analysis.
Total proteins were isolated by sonication of cell in lysis buffer composed of 50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, pH 8, 2.5 mM EGTA, pH 7.4, 0.1% Tween 20, 10% glycerol, 0.1 mM sodium orthovanadate, 1 mM sodium fluoride, 10 mM β-glycerophosphate, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), and 1× complete protease inhibitor cocktail (Roche).
A 10- to 20-μg sample of proteins was resolved on 8% SDS-PAGE and transferred to a nitrocellulose membrane (0.45-μm pore size) (Amersham). Mouse anti-human HIF-1α (610958, clone 54; BD Transduction Laboratories) and mouse anti-human heat shock cognate 70 (HSC70) (B-6, sc-7298; Santa Cruz Biotechnology), used as loading controls, were diluted, respectively, at ratios of 1:1,000 and 1:10,000. A second mouse antibody (Dako) was used at a dilution of 1:1,000. Detection was performed using enhanced chemiluminescence (ECL; Fisher Scientific, Pierce).
RNA isolation, reverse transcription, and quantitative real-time PCR.
Total RNA was extracted from airway epithelial cells using the RNeasy minikit (Qiagen). After DNase treatment using RQ1 RNase-free DNase (Promega), 1 μg of RNA was reverse transcribed into cDNA using oligo(dT) primer and avian myeloblastosis virus (AMV) reverse transcriptase (Promega). Quantitative real-time PCR was performed using the FastStart TaqMan probe master mix (Roche) and the PTC-200 thermocycler (MJ Research). The following primer pairs were used at a final concentration of 200 nM with the corresponding Roche probe number in parentheses at a final concentration of 200 nM: HPRT1-F, 5′-TGACCTTGATTTATTTTGCATACC-3′, and HPRT1-R, 5′-CGAGCAAGACGTTCAGTCCT-3′ (no. 73); HIF-1α-F, 5′-TTTTTCAAGCAGTAGGAATTGGA-3′, and HIF-1α-R, 5′-GTGATGTAGTAGCTGCATGATCG-3′ (no. 66); and HKII-F, 5′-TCCCCTGCCACCAGACTA-3′, and HKII-R, 5′-TGGACTTGAATCCCTTGGTC-3′ (no. 54). The relative quantification of mRNA levels was calculated with the 2−ΔΔCT method using HPRT-1 threshold cycle (CT) values for normalization.
Cytotoxicity assay.
The release of lactate dehydrogenase (LDH) into cell culture supernatants was measured using an LDH cytotoxicity detection kit (Roche) according to the manufacturer's instructions.
Statistical analysis.
Three independent biological replicates were performed for all experiments described in this paper. Statistical analysis was performed using a two-tailed unpaired Student's t test. Differences were considered significant if the P value was ≤0.05.
RESULTS
P. aeruginosa cell-free culture supernatants downregulate HIF-1α protein levels.
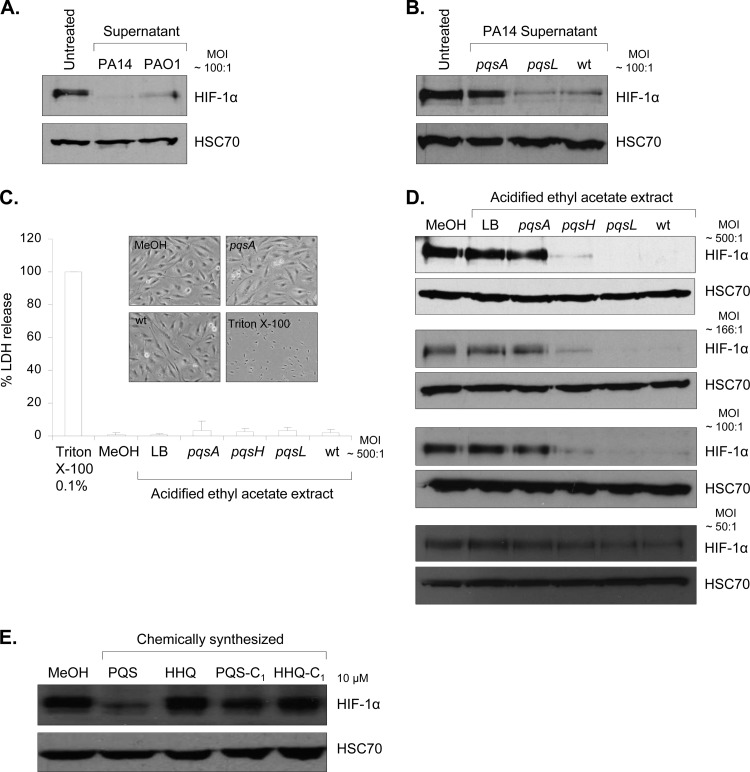
In order to identify the impact of extracellular molecules produced by P. aeruginosa on HIF-1α protein levels, cell-free culture supernatants were coincubated with IB3-1 airway epithelial cells. We found that culture supernatants from both P. aeruginosa strains PA14 and PAO1 led to a marked repression of HIF-1α protein levels (Fig. 1A). This observation was unexpected, as previously we showed that direct coculture of P. aeruginosa with IB3-1 cells induced HIF-1α protein stabilization (26) and to date bacterial secreted compounds have only been shown to induce HIF-1α protein levels (15, 18).
Fig 1.
P. aeruginosa AQ signaling molecules, including PQS, downregulate HIF-1α protein levels. (A) Expression of HIF-1α and HSC70 (loading control) proteins in human airway epithelial cells (IB3-1 cells) in untreated control conditions or after incubation with P. aeruginosa cell-free culture supernatants from PA14 or PAO1 strains (expressing AQs) at a dilution corresponding to an MOI of 100:1 for 16 h. (B) Expression of HIF-1α and HSC70 (loading control) proteins in human airway epithelial cells (IB3-1 cells) in untreated control conditions or after incubation with P. aeruginosa cell-free culture supernatants from a PA14 pqsA mutant (impaired AQ production), a PA14 pqsL mutant (producing AQs and overproducing PQS) or PA14 wt at a dilution corresponding to an MOI of 100:1 for 16 h. (C) Release of LDH in cell culture medium of airway epithelial cells (IB3-1) treated with 0.1% Triton X-100, methanol (MeOH), or acidified ethyl acetate extracts from LB (control) or from cell-free culture supernatants of P. aeruginosa PA14 pqsA, pqsH (producing all AQs except PQS), or pqsL mutants, or PA14 wt at a dilution corresponding to an MOI of 500:1 for 16 h. Cytotoxicity is expressed as a percentage of the total amount of LDH released from airway epithelial cells treated with 0.1% Triton X-100, given the arbitrary percentage of 100. Phase-contrast microscopy of IB3-1 cells treated with methanol (MeOH) or 0.1% Triton X-100 or acidified ethyl acetate extracts from culture supernatants of P. aeruginosa PA14 pqsA or PA14 wt at a dilution for 16 h corresponding to an MOI of 500:1. Original magnification, ×40. (D) Expression of HIF-1α and HSC70 (loading control) proteins in airway epithelial cells (IB3-1 cells) treated with methanol (MeOH) or acidified ethyl acetate extracts from LB (control) or from cell-free culture supernatants of P. aeruginosa PA14 pqsA, pqsH, or pqsL mutants or PA14 wt at a dilution corresponding to an MOI of 500:1, 166:1, 100:1 and 50:1 for 16 h. (E) Expression of HIF-1α and HSC-70 (loading control) proteins in airway epithelial cells (IB3-1 cells) treated with methanol (MeOH) or chemically synthesized PQS, HHQ or PQS and HHQ lacking its alkyl chain, termed, respectively, PQS-C1 and HHQ-C1, at 10 μM for 16 h.
P. aeruginosa cell-free culture supernatants downregulate HIF-1α protein levels in a psqA-dependent manner.
To further investigate this novel observation, we targeted the 2-alkyl-4-quinolone (AQ) signaling pathway since the production of extracellular factors by P. aeruginosa is significantly dependent on this intercellular signaling system and AQ signal molecules have been recently shown to modulate the expression of two regulators of the HIF-1 pathway (23, 24), NF-κB (43, 52) and early growth factor 1 (EGR1) (37, 51). Therefore, IB3-1 cells were incubated with culture supernatants from a P. aeruginosa PA14 wt strain, an isogenic pqsA mutant strain impaired in AQ production (PA14 pqsA) (10), and an isogenic pqsL mutant strain (PA14 pqsL), known to produce all AQs and to overproduce the principal AQ signal molecule, PQS (8). None of the strains displayed a growth effect, as shown by growth curve analysis (data not shown). Subsequent analysis of HIF-1α protein levels revealed that culture supernatant from the pqsA mutant was unable to repress HIF-1α protein levels compared to supernatant from the pqsL mutant or from the PA14 wt strain (Fig. 1B). This suggests for the first time that P. aeruginosa AQ signal molecules may play a role in the inhibition of HIF-1α protein accumulation in airway epithelial cells.
P. aeruginosa AQ signal molecules downregulate HIF-1α protein levels.
As the PA14 pqsA mutant is also impaired in the production of several other extracellular molecules, such as phenazines and pyocyanin (10), a specific extraction of AQs by acidified ethyl acetate was performed. The relative quantity of PQS present in the extracts was estimated by TLC in comparison with synthetic PQS and HHQ standards (see Fig. S1 in the supplemental material). The PA14 wt extract contained a concentration of PQS of approximately 15 μM; the PA14 pqsL mutant contained a PQS concentration of about 45 μM, whereas as expected the pqsA mutant contained no PQS (Fig. S1). Furthermore, PQS was not detected in extracts from a pqsH mutant, which does not possess the enzymatic activity required to convert HHQ into PQS (11) (Fig. S1).
To rule out a general stress response caused by cellular damage, the potential cytotoxic effect of the extracts was assessed at a dilution corresponding to the highest MOI of 500:1. No increased release of lactate dehydrogenase (LDH) in the cell culture medium (Fig. 1C) was detected in response to all the extracts, and no significant changes in cell morphology were observed, as illustrated with IB3-1 cell imaging in the presence of extracts from the PA14 pqsA mutant and PA14 wt (Fig. 1C).
All AQ extracts were subsequently tested for their ability to reduce HIF-1α protein levels in IB3-1 cells.
AQ extracts from PA14 wt and the PA14 pqsL mutant led to a marked concentration-dependent repression of HIF-1α protein levels compared to control conditions (methanol-treated cells or extract from LB medium alone) (Fig. 1D). Extracts from a pqsH mutant only partially inhibited HIF-1α protein levels, suggesting a role for PQS, whereas extract from the pqsA mutant was unable to repress HIF-1α protein levels (Fig. 1D). In contrast, protein levels of the second HIF-1 protein subunit, HIF-1β, were not affected by any of the AQ extracts tested in IB3-1 cells (see Fig. S2 in the supplemental material). As CFTR-corrected IB3-1 cells (S9 cells) and human lung epithelial cells (A549 cells) respond similarly (data not shown), we conclude that HIF-1α protein repression was not linked to the CF status and CFTR mutation(s).
PQS is directly involved in the downregulation of HIF-1α protein levels.
To decipher which P. aeruginosa AQ(s) was responsible for the inhibition of HIF-1α protein accumulation, the two major and active AQs detected in the sputum of CF patients (5, 16, 29), PQS and its precursor HHQ, previously chemically synthesized (41), were tested for their ability to modulate HIF-1α protein levels in IB3-1 cells. A physiologically relevant concentration of 10 μM each synthetic AQ was used. Whereas HHQ did not significantly affect HIF-1α protein levels, PQS strongly repressed HIF-1α protein levels in IB3-1 airway epithelial cells (Fig. 1E). Importantly, this repression was still significant for a PQS concentration of 1 μM (data not shown). The alkyl side chains of PQS and HHQ have been shown to be fundamental in modulating microbial behavior (41). Therefore, the effect of PQS and HHQ analogues lacking the alkyl chains (41), termed PQS-C1 and HHQ-C1, respectively, on HIF-1α protein levels was tested. PQS-mediated HIF-1α protein repression was abrogated in epithelial cells in response to PQS-C1 (Fig. 1E), suggesting that the alkyl side chain of PQS is important for the downregulation of HIF-1α. Similar to HHQ, HHQ-C1 had no effect on HIF-1α protein levels.
Altogether, AQ-dependent downregulation of HIF-1α protein could at least in part be attributed to PQS. However, as extract from a P. aeruginosa PA14 pqsH mutant, which produces AQs, including HHQ, but not PQS, was also able to partially inhibit HIF-1α protein accumulation, an as-yet-unidentified but distinct AQ might play a role similar to that of PQS.
Under hypoxic conditions, P. aeruginosa AQ signaling molecules downregulate HIF-1α protein levels and repress HIF-1 target gene expression.
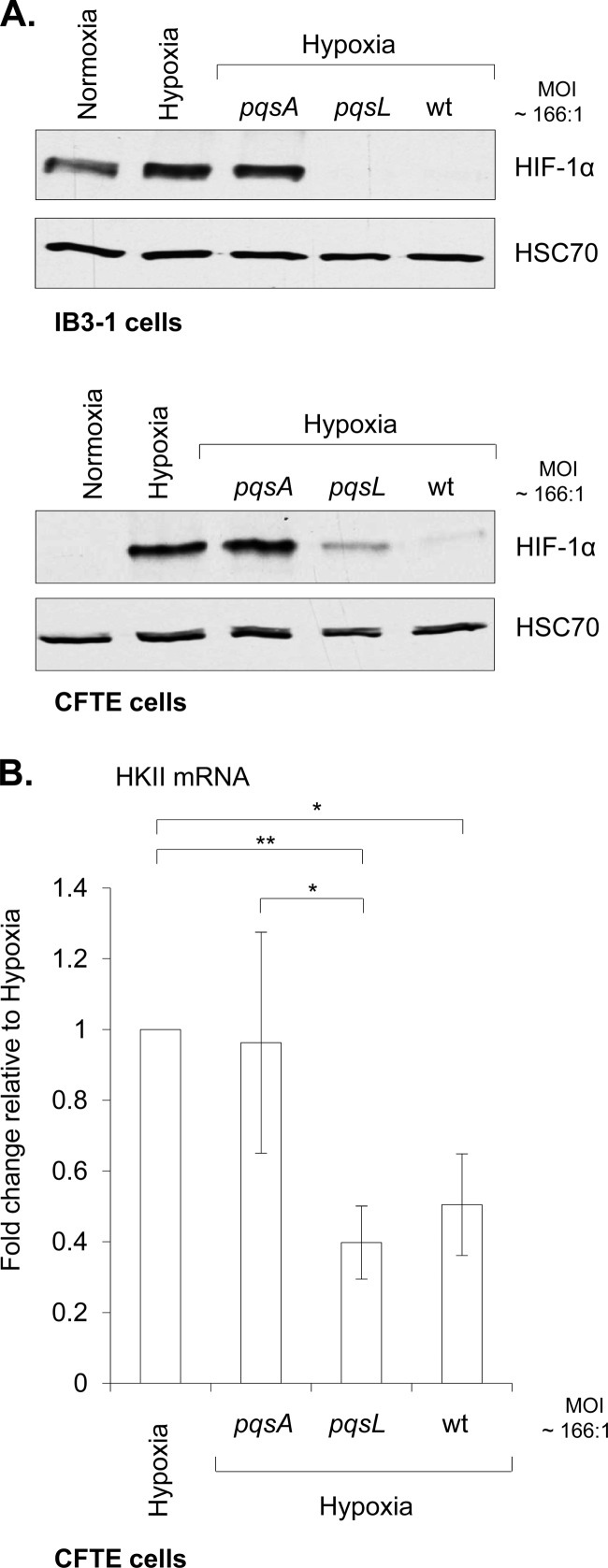
Downregulation of the protein subunit HIF-1α levels by AQ signal molecules could have dramatic consequences for the cell. This is particularly relevant in the molecular and cellular management of hypoxic stress, such as that encountered in chronically P. aeruginosa-infected lungs of CF patients (2, 56). In order to determine if P. aeruginosa AQs were able to repress hypoxia-mediated HIF-1α stabilization, airway epithelial cells were exposed to hypoxic conditions (1% O2 for 16 h) with or without P. aeruginosa extracts used at a dilution corresponding to a physiologically relevant PQS concentration (ca. 3 μM for pqsL extract, equivalent to an MOI of 166:1). AQ signal molecules (PA14 pqsL and wt extracts) were able to strongly downregulate hypoxia-mediated HIF-1α stabilization in both IB3-1 and CFTE cells (Fig. 2A). Consistent with the role for AQs in mediating this repression, extracts from the pqsA mutant failed to influence HIF-1α stabilization in either cell line under hypoxia (Fig. 2A). Surprisingly, HIF-1α was not strongly stabilized by hypoxia in the IB3-1 airway epithelial cells compared to the CFTE airway epithelial cell line (Fig. 2A). The difference between these two cell lines may be explained by the high levels of HIF-1α protein already present under normoxic conditions in IB3-1 cells.
Fig 2.
P. aeruginosa AQ signaling molecules downregulate HIF-1α protein levels under hypoxic conditions: repression of the hypoxia-mediated HKII induction. (A) Expression of HIF-1α and HSC70 (loading control) proteins in airway epithelial IB3-1 or CFTE cells in normoxic conditions (Normoxia) or hypoxic conditions (Hypoxia), alone or in association with acidified ethyl acetate extracts from cell-free culture supernatants of P. aeruginosa PA14 pqsA or pqsL mutants or PA14 wt at a dilution corresponding to an MOI of 166:1 for 16 h. (B) Expression of HKII mRNA in airway epithelial cells (CFTE cells) in hypoxic conditions (Hypoxia), alone or in association with acidified ethyl acetate extracts from cell-free culture supernatants of P. aeruginosa PA14 pqsA or pqsL mutants or PA14 wt at a dilution corresponding to an MOI of 166:1 for 16 h. HKII mRNA fold change is expressed as mean ± standard deviation (n = 3) and relative to hypoxic conditions, given the arbitrary value of 1. Two-tailed unpaired Student's t test was performed (*, P value ≤ 0.05; **, P value ≤ 0.01).
As proof of concept, we investigated if a downstream target of HIF-1 was in turn influenced by AQ extracts during hypoxia. Airway epithelial cells are known to be tolerant to hypoxia due to the rapid implementation of adaptive strategies, including the increase of glucose metabolism in order to preserve ATP supply by upregulating the expression of glycolytic enzymes. One of these, the hexokinase II (HKII), has been already shown to be induced in the human lung epithelial cell line A549 under hypoxic conditions (42). Therefore, evaluation of HKII expression was undertaken in CFTE cells under hypoxic conditions in the presence of P. aeruginosa PA14 pqsA, pqsL, and wt cell extracts. By comparison to hypoxic conditions, while extract from the pqsA mutant did not affect the induction of HKII under hypoxic conditions (Fig. 2B), the induction of this important HIF-1 target gene was strongly and significantly repressed in the presence of either P. aeruginosa PA14 pqsL or wt extracts (Fig. 2B). Repression of HKII mRNA expression by the pqsL extract was still significant in comparison with that by the pqsA extract under hypoxia. Taken together, these results suggest that P. aeruginosa AQ signaling molecules, by inhibiting HIF-1α protein subunit accumulation under hypoxia, repress induction of the hypoxia-mediated HIF-1 target, HKII.
P. aeruginosa AQ signaling molecules do not affect HIF-1α mRNA level.
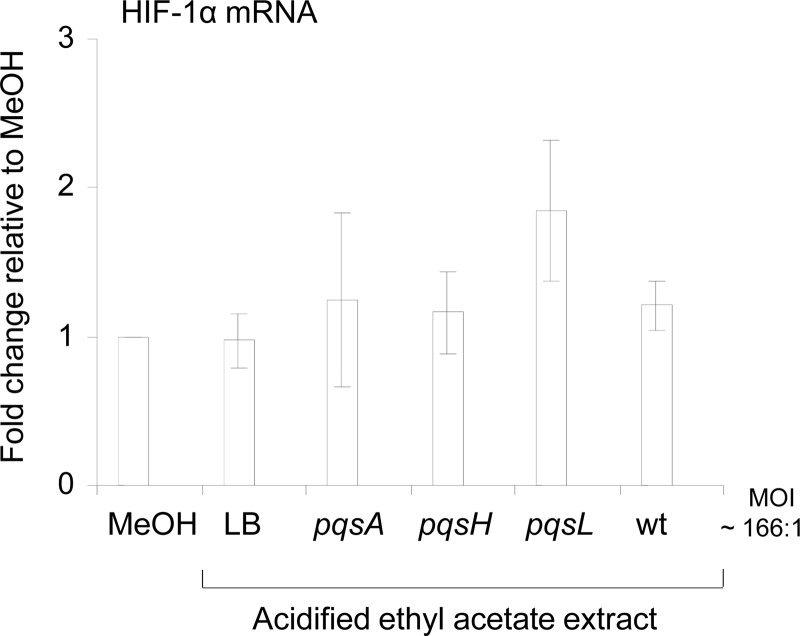
In order to investigate the mechanism by which P. aeruginosa AQ signal molecules repressed the HIF-1α protein level, we first investigated if HIF-1α expression was modulated at the mRNA level in IB3-1 cells. The HIF-1α mRNA level was not significantly affected in response to P. aeruginosa extracts compared to the control methanol conditions (Fig. 3), indicating that downregulation of HIF-1α protein by P. aeruginosa AQs is not due to a modulation of its transcript expression.
Fig 3.
P. aeruginosa AQ signaling molecules do not affect HIF-1α mRNA transcript levels. Expression of HIF-1α mRNA in airway epithelial cells (IB3-1 cells) treated with methanol (MeOH) or acidified ethyl acetate extracts from LB (control) or from cell-free culture supernatants of P. aeruginosa PA14 pqsA, pqsH, or pqsL mutants or PA14 wt at a dilution corresponding to an MOI of 166:1 for 16 h. HIF-1α mRNA fold change is expressed as mean ± standard deviation (n = 3) and relative to methanol control conditions, given the arbitrary value of 1.
Oxidative stress is not involved in HIF-1α protein downregulation by P. aeruginosa AQ extracts.
As PQS has recently been shown to have an antioxidant/prooxidant activity (19) and since oxidative stress and reactive oxygen species (ROS) are involved in the modulation of HIF-1α protein expression (50, 54), the potential role of ROS production in P. aeruginosa AQ-mediated HIF-1α downregulation was assessed by using both antioxidant and ROS scavenger compounds. However, treatments of IB3-1 airway epithelial cells by either the antioxidant N-acetyl cysteine (NAC) (data not shown) or the ROS scavenger dimethyl sulfoxide (DMSO) (data not shown) were not able to prevent the downregulation of HIF-1α protein levels by P. aeruginosa AQ extracts. This suggests that ROS and oxidative stress are not involved in the mediation of HIF-1α destabilization by P. aeruginosa AQs.
P. aeruginosa AQ signaling molecules lead to HIF-1α protein degradation via a 26S proteasome-dependent mechanism but in a PHD- and calpain-independent manner.
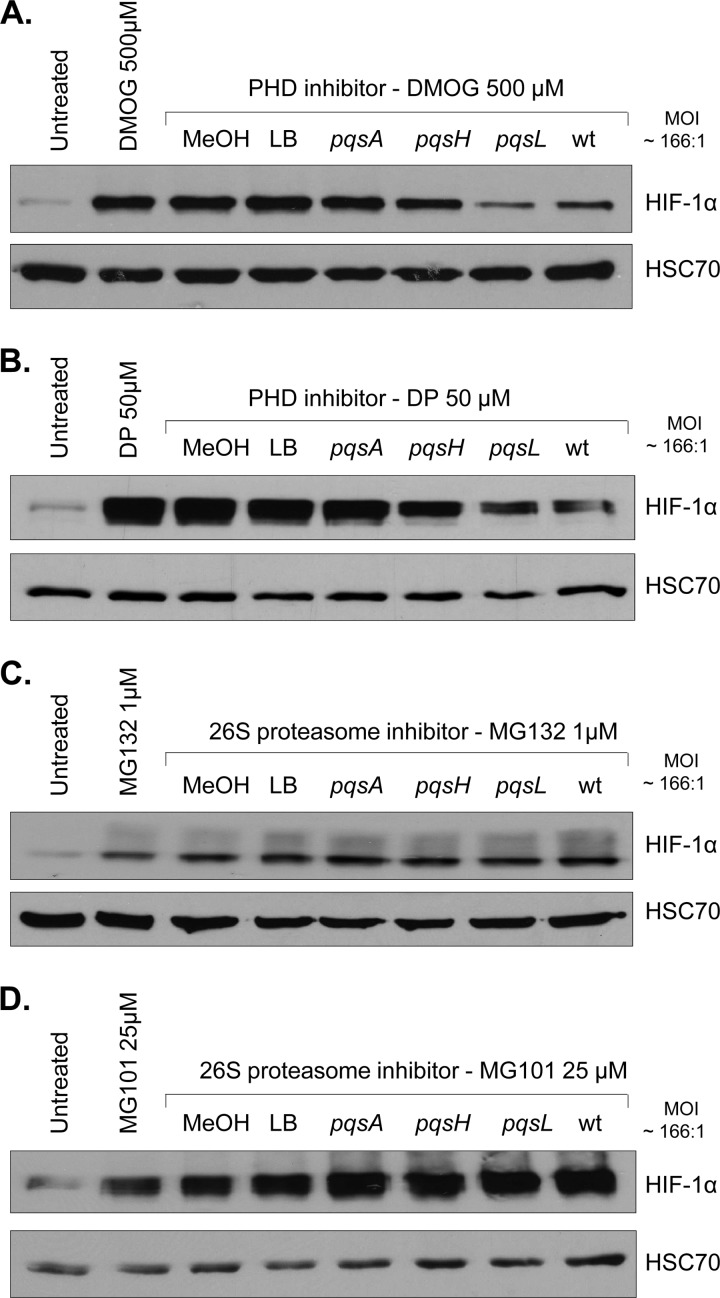
The best-known mechanism for controlling HIF-1α expression is protein degradation via the 26S proteasome machinery, raising the possibility that P. aeruginosa AQ-mediated HIF-1α protein repression may act at this posttranslational level. However, before it can be degraded by the proteasome, HIF-1α protein must first be hydroxylated by PHD enzymes and subsequently bind with pVHL (21, 46). In order to study the potential involvement of the PHD pathway in AQ-mediated HIF-1α protein repression, two chemical PHD inhibitors were utilized. As expected, both dimethyloxaloylglycine (DMOG) (Fig. 4A) and 2,2′-dipyridyl (DP) (Fig. 4B) led to the stabilization of HIF-1α in IB3-1 cells compared to untreated control cells. HIF-1α stabilization by PHD inhibitors was maintained in the presence of methanol, LB, or pqsA extracts. However, HIF-1α protein was markedly less stabilized by PHD inhibitors in the presence of the PA14 pqsL and PA14 wt extracts and less stabilized in the presence of the PA14 pqsH extract. Thus, these results suggest that P. aeruginosa AQ-mediated HIF-1α protein repression occurs independently of the PHD pathway. Moreover, the same results were observed in CFTE cells in the presence of the DP molecule (see Fig. S3 in the supplemental material).
Fig 4.
P. aeruginosa AQ signaling molecules mediate HIF-1α degradation through a PHD-independent but 26S proteasome-dependent mechanism. Expression of HIF-1α and HSC70 (loading control) proteins in airway epithelial cells (IB3-1 cells) untreated or treated with the PHD inhibitors DMOG (500 μM) (A) and DP (50 μM) (B) or with the 26S proteasome inhibitors MG132 (1 μM) (C) and MG101 (25 μM) (D), alone or in association with methanol (MeOH) or acidified ethyl acetate extracts from LB (control) or from cell-free culture supernatants of P. aeruginosa PA14 pqsA, pqsH, or pqsL mutants or PA14 wt at a dilution corresponding to an MOI of 166:1 for 16 h.
As it has been reported that degradation of the HIF-1α protein can also occur through an alternative pathway requiring calpain activation (33, 58), the involvement of this pathway was investigated. However, in the presence of two calpain inhibitors, N-acetyl-Leu-Leu-methional (ALLM) and the calpastatin peptide, HIF-1α protein was still completely downregulated by P. aeruginosa AQ signaling molecules (see Fig. S4 in the supplemental material).
However, it has been reported that degradation of the HIF-1α protein can still occur via the 26S proteasome independently of the pVHL/PHD or calpain enzymes (57). Therefore, the effect of P. aeruginosa extracts on HIF-1α protein levels in IB3-1 cells was investigated in the presence of 26S proteasome inhibitors. Interestingly, Z-Leu-Leu-Leu-al (MG132) (Fig. 4C) and N-acetyl-Leu-Leu-norleucinal (MG101) (Fig. 4D), two 26S proteasome inhibitors, stabilized HIF-1α protein in the presence of all extracts, and similar results were observed with CFTE cells with MG132 (see Fig. S3 in the supplemental material). Therefore, P. aeruginosa AQ extracts, in the presence of the 26S proteasome inhibitors, were no longer able to repress HIF-1α protein levels. Thus, we can conclude that P. aeruginosa AQs degrade HIF-1α protein through the 26S proteasome pathway but independently of the PHD and calpain pathways.
DISCUSSION
Activation of HIF-1 signaling and subsequent induction of HIF-1 target genes postinfection has emerged as a key step in the activation of innate immunity and the proinflammatory response to infection (39, 40). Here we demonstrate that P. aeruginosa-secreted AQ intercellular signaling molecules, and in particular PQS, can downregulate HIF-1α protein levels. This is the first evidence of bacterial compounds having a negative impact on HIF-1α levels; all previous data have shown an accumulation of HIF-1α protein in response to bacterial compounds (siderophore and LPS) (15, 18) and to direct contact with bacteria, including P. aeruginosa (3, 15, 18, 25, 26, 39, 40, 44, 49, 55).
Previously, we showed that the alkyl side chain was crucial for PQS in its role in modulating microbial behavior (41). This side chain was also fundamental to AQ-mediated HIF-1α destabilization, indicating the importance of this alkyl chain in signal transduction. A steep potency curve for AQ-mediated HIF-1α destabilization was observed, which could point to the fact that a threshold for the detection of AQs might be necessary in order to degrade HIF-1α. Threshold levels of AQs could be reached during chronic infection, which might lead to the destabilization of HIF-1α and subsequent modulation of the immune response (Fig. 5).
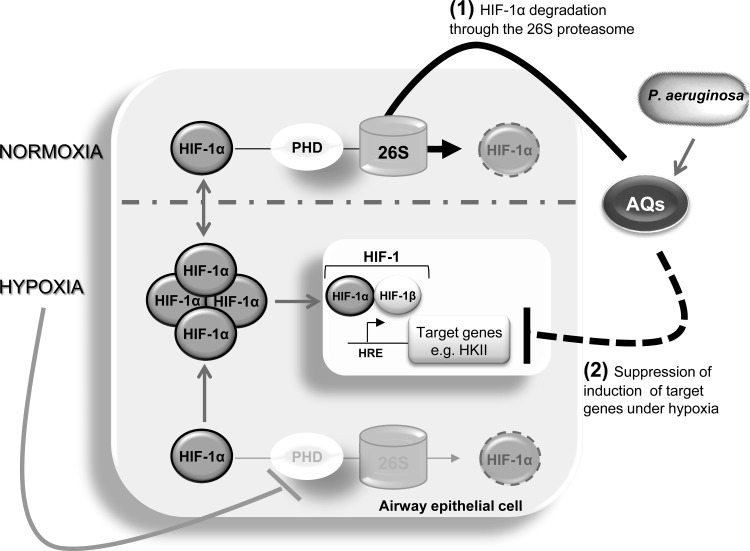
Fig 5.
Model: AQ-mediated HIF-1 signaling. Under normal levels of oxygen (normoxia), HIF-1α protein subunit is constitutively synthesized by the cells but quickly degraded through the PHD/26S proteasomal degradation pathway. Reduced oxygen levels (hypoxia) lead to the inhibition of the PHD enzymes that prevent HIF-1α degradation, leading to accumulation of HIF-1α. Upon infection, P. aeruginosa produces an arsenal of molecules involved in its pathogenicity, including the 2-alkyl-4-quinolone (AQ) signaling molecules. Here, we have shown that AQs lead to the degradation of the HIF-1α protein through the 26S proteasome machinery (1), which in turn lead to the suppression of induction of the hexokinase II (HKII) under hypoxia (2).
Destabilization of HIF-1α by P. aeruginosa signaling molecules may represent an adaptive strategy by the pathogen to counter the deleterious effects of immune activation arising from HIF-1α stabilization upon initial contact. This is particularly significant in the context of CF disease, as PQS has been found in the sputum of CF patients (16). Moreover, HHQ and PQS have been shown to have immunomodulatory activity. Kim and others demonstrated that both HHQ and PQS downregulated the immune response through the NF-κB pathway in mouse macrophage cell lines (23, 24), while a non-AQ-producing PA14 pqsA mutant was shown to be avirulent in mouse burn wound infection models (10). In addition, it has been shown that P. aeruginosa is capable of recognizing activation of HIF-1α in epithelial cells (38). Activation of HIF-1α was shown to result in the secretion of an adenosine-type compound from host cells that triggers the production of PQS-regulated PA-I lectin in P. aeruginosa (38). Therefore, subsequent destabilization of HIF-1α by P. aeruginosa AQ signaling molecules may represent a microbial countermeasure to the host response during infection.
HIF-1α destabilization occurred through its specific degradation via the ubiquitin 26S proteasome proteolytic pathway but independently of the hydroxylation mediated by PHD or the calpain pathway. This interesting aspect of the mechanism through which P. aeruginosa AQ signal molecules degrade HIF-1α remains to be elucidated and warrants further characterization. However, Kruppel-like factor 2 (KLF2) could be considered a potential candidate in light of recent reports revealing its role in PHD-independent HIF-1α proteasomal degradation, through direct disruption of the HSP90/HIF-1α complex (22). In addition, signaling pathways of KLF2 and HIF-1 have been recently shown to be interconnected upon bacterial infection (30, 31). A second consideration could be direct interference with the ubiquitination stage of protein degradation, as evidence has already revealed that microbial compounds could target this pathway (45). Indeed, the secreted virulence factor Cif from P. aeruginosa has been shown to increase the degradation of CFTR in lysosomes, through the reduction of USP10-mediated deubiquitination of CFTR (1). Therefore, further investigation is warranted in order to fully understand the mechanisms through which P. aeruginosa influences the host response at the site of infection and, particularly, to better characterize the role of the ubiquitination in this process.
Development of antagonists of Pseudomonas AQs, which may prevent HIF-1 degradation and may decrease the production of Pseudomonas virulence factors, could be considered a promising new strategy in the treatment of P. aeruginosa infection and in particular in CF disease. Indeed, boosting HIF-1 signaling in order to fight against infection is seen as an emerging therapeutic strategy. Two compounds, mimosine and AKB-4924, have been shown to stimulate host defense by enhancing HIF-1α stabilization in mouse models (35, 60). However, the balance between activation and inhibition should be perfectly controlled, as HIF-1 is highly involved in cancer biology and as such has emerged, in addition to some of its target genes, including HKII, as a new target for anticancer therapy (47). Therefore, understanding the molecular mechanisms through which microbial bioactive compounds modulate the function of this key human signal is crucial to its exploitation for future innovative therapies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Pat Higgins for excellent technical support. We also thank Dmitri B. Papkovsky, Alexander V. Zhdanov, Grzegorz Jasionek, and Nicolas B. Borchert (from the Biochemistry Department, University College Cork, Cork, Ireland) for the use of the hypoxic chamber (Coy Scientific).
This research was supported in part by grants awarded by the European Commission (FP7-KBBE-2012-6, CP-TP-312184; FP7-KBBE-2012-6, 311975; OCEAN 2011-2, 287589; MTKD-CT-2006-042062, O36314), Science Foundation Ireland (07/IN.1/B948; 08/RFP/GEN1295; 08/RFP/GEN1319; 09/RFP/BMT2350), the Department of Agriculture and Food (DAF RSF 06 321; DAF RSF 06 377; FIRM 08/RDC/629), the Irish Research Council for Science, Engineering and Technology (RS/2010/2413; 05/EDIV/FP107), the Health Research Board (RP/2006/271; RP/2007/290; HRA/2009/146), the Environmental Protection Agency (EPA2006-PhD-S-21; EPA2008-PhD-S-2), the Marine Institute (Beaufort Award C2CRA 2007/082), and the Higher Education Authority of Ireland (PRTLI3; PRTLI4).
Footnotes
Published ahead of print 4 September 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Bomberger JM, et al. 2011. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 7:e1001325 doi:10.1371/journal.ppat.1001325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boucher RC. 2004. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 23:146–158 [DOI] [PubMed] [Google Scholar]
- 3. Cane G, et al. 2010. HIF-1alpha mediates the induction of IL-8 and VEGF expression on infection with Afa/Dr diffusely adhering E. coli and promotes EMT-like behaviour. Cell. Microbiol. 12:640–653 [DOI] [PubMed] [Google Scholar]
- 4. Coggan KA, Wolfgang MC. 2012. Global regulatory pathways and cross-talk control Pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr. Issues Mol. Biol. 14:47–70 [PubMed] [Google Scholar]
- 5. Collier DN, et al. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 215:41–46 [DOI] [PubMed] [Google Scholar]
- 6. Cramer T, et al. 2003. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112:645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darch SE, West SA, Winzer K, Diggle SP. 2012. Density-dependent fitness benefits in quorum-sensing bacterial populations. Proc. Natl. Acad. Sci. U. S. A. 109:8259–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Argenio DA, Calfee MW, Rainey PB, Pesci EC. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dev A, Iyer S, Razani B, Cheng G. 2011. NF-kappaB and innate immunity. Curr. Top. Microbiol. Immunol. 349:115–143 [DOI] [PubMed] [Google Scholar]
- 10. Deziel E, et al. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol. Microbiol. 55:998–1014 [DOI] [PubMed] [Google Scholar]
- 11. Deziel E, et al. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. U. S. A. 101:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diggle SP, et al. 2007. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol. 14:87–96 [DOI] [PubMed] [Google Scholar]
- 13. Eltzschig HK, Carmeliet P. 2011. Hypoxia and inflammation. N. Engl. J. Med. 364:656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher MP, Diggle SP, Camara M, Williams P. 2007. Biosensor-based assays for PQS, HHQ and related 2-alkyl-4-quinolone quorum sensing signal molecules. Nat. Protoc. 2:1254–1262 [DOI] [PubMed] [Google Scholar]
- 15. Frede S, Stockmann C, Freitag P, Fandrey J. 2006. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem. J. 396:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guina T, Purvine SO, Yi EC, Eng J, Goodlett DR, Aebersold R, Miller SI. 2003. Quantitative proteomic analysis indicates increased synthesis of a quinolone by Pseudomonas aeruginosa isolates from cystic fibrosis airways. Proc. Natl. Acad. Sci. U. S. A. 100:2771–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hao Y, et al. 2012. Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and metaplasia and mucus hypersecretion by inactivating the transcriptional factor FoxA2. Cell. Microbiol. 14:401–415 [DOI] [PubMed] [Google Scholar]
- 18. Hartmann H, et al. 2008. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 134:756–767 [DOI] [PubMed] [Google Scholar]
- 19. Haussler S, Becker T. 2008. The pseudomonas quinolone signal (PQS) balances life and death in Pseudomonas aeruginosa populations. PLoS Pathog. 4:e1000166 doi:10.1371/journal.ppat.1000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heeb S, et al. 2011. Quinolones: from antibiotics to autoinducers. FEMS Microbiol. Rev. 35:247–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jaakkola P, et al. 2001. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472 [DOI] [PubMed] [Google Scholar]
- 22. Kawanami D, et al. 2009. Kruppel-like factor 2 inhibits hypoxia-inducible factor 1alpha expression and function in the endothelium. J. Biol. Chem. 284:20522–20530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim K, Kim SH, Lepine F, Cho YH, Lee GR. 2010. Global gene expression analysis on the target genes of PQS and HHQ in J774A.1 monocyte/macrophage cells. Microb. Pathog. 49:174–180 [DOI] [PubMed] [Google Scholar]
- 24. Kim K, et al. 2010. HHQ and PQS, two Pseudomonas aeruginosa quorum-sensing molecules, down-regulate the innate immune responses through the nuclear factor-kappaB pathway. Immunology 129:578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koury J, et al. 2004. Persistent HIF-1alpha activation in gut ischemia/reperfusion injury: potential role of bacteria and lipopolysaccharide. Shock 22:270–277 [DOI] [PubMed] [Google Scholar]
- 26. Legendre C, Mooij MJ, Adams C, O'Gara F. 2011. Impaired expression of hypoxia-inducible factor-1alpha in cystic fibrosis airway epithelial cells—a role for HIF-1 in the pathophysiology of CF? J. Cyst. Fibros. 10:286–290 [DOI] [PubMed] [Google Scholar]
- 27. Lepine F, Milot S, Deziel E, He J, Rahme LG. 2004. Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa. J. Am. Soc. Mass Spectrom. 15:862–869 [DOI] [PubMed] [Google Scholar]
- 28. Liberati NT, et al. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. U. S. A. 103:2833–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Machan ZA, Taylor GW, Pitt TL, Cole PJ, Wilson R. 1992. 2-Heptyl-4-hydroxyquinoline N-oxide, an antistaphylococcal agent produced by Pseudomonas aeruginosa. J. Antimicrob. Chemother. 30:615–623 [DOI] [PubMed] [Google Scholar]
- 30. Mahabeleshwar GH, et al. 2011. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity 34:715–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahabeleshwar GH, et al. 2012. A myeloid hypoxia-inducible factor 1alpha-Kruppel-like factor 2 pathway regulates gram-positive endotoxin-mediated sepsis. J. Biol. Chem. 287:1448–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mayer ML, Sheridan JA, Blohmke CJ, Turvey SE, Hancock RE. 2011. The Pseudomonas aeruginosa autoinducer 3O-C12 homoserine lactone provokes hyperinflammatory responses from cystic fibrosis airway epithelial cells. PLoS One 6:e16246 doi:10.1371/journal.pone.0016246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nanduri J, et al. 2009. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc. Natl. Acad. Sci. U. S. A. 106:1199–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nizet V, Johnson RS. 2009. Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 9:609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okumura CY, et al. 2012. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J. Mol. Med. (Berl.) 90:1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Sullivan BP, Freedman SD. 2009. Cystic fibrosis. Lancet 373:1891–1904 [DOI] [PubMed] [Google Scholar]
- 37. Patel N, Kalra VK. 2010. Placenta growth factor-induced early growth response 1 (Egr-1) regulates hypoxia-inducible factor-1alpha (HIF-1alpha) in endothelial cells. J. Biol. Chem. 285:20570–20579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel NJ, et al. 2007. Recognition of intestinal epithelial HIF-1 alpha activation by Pseudomonas aeruginosa. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G134–G142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peyssonnaux C, et al. 2008. Critical role of HIF-1alpha in keratinocyte defense against bacterial infection. J. Invest. Dermatol. 128:1964–1968 [DOI] [PubMed] [Google Scholar]
- 40. Peyssonnaux C, et al. 2005. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 115:1806–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reen FJ, et al. 2011. The Pseudomonas quinolone signal (PQS), and its precursor HHQ, modulate interspecies and interkingdom behaviour. FEMS Microbiol. Ecol. 77:413–428 [DOI] [PubMed] [Google Scholar]
- 42. Riddle SR, et al. 2000. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L407–L416 [DOI] [PubMed] [Google Scholar]
- 43. Rius J, et al. 2008. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453:807–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rupp J, et al. 2007. Chlamydia pneumoniae directly interferes with HIF-1alpha stabilization in human host cells. Cell. Microbiol. 9:2181–2191 [DOI] [PubMed] [Google Scholar]
- 45. Rytkonen A, Holden DW. 2007. Bacterial interference of ubiquitination and deubiquitination. Cell Host Microbe 1:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schofield CJ, Ratcliffe PJ. 2005. Signalling hypoxia by HIF hydroxylases. Biochem. Biophys. Res. Commun. 338:617–626 [DOI] [PubMed] [Google Scholar]
- 47. Semenza GL. 2003. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3:721–732 [DOI] [PubMed] [Google Scholar]
- 48. Shao Z, Zhang Y, Ye Q, Saldanha JN, Powell-Coffman JA. 2010. C. elegans SWAN-1 binds to EGL-9 and regulates HIF-1-mediated resistance to the bacterial pathogen Pseudomonas aeruginosa PAO1. PLoS Pathog. 6:e1001075 doi:10.1371/journal.ppat.1001075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sharma M, et al. 2011. HIF-1alpha is involved in mediating apoptosis resistance to Chlamydia trachomatis-infected cells. Cell. Microbiol. 13:1573–1585 [DOI] [PubMed] [Google Scholar]
- 50. Sommani P, et al. 2007. Inhibitory effect of 6-formylpterin on HIF-1alpha protein accumulation. Biol. Pharm. Bull. 30:2181–2184 [DOI] [PubMed] [Google Scholar]
- 51. Sperandio S, et al. 2009. The transcription factor Egr1 regulates the HIF-1alpha gene during hypoxia. Mol. Carcinog. 48:38–44 [DOI] [PubMed] [Google Scholar]
- 52. van Uden P, Kenneth NS, Rocha S. 2008. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem. J. 412:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang GL, Jiang BH, Rue EA, Semenza GL. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U. S. A. 92:5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wellman TL, et al. 2004. Nitric oxide and reactive oxygen species exert opposing effects on the stability of hypoxia-inducible factor-1alpha (HIF-1alpha) in explants of human pial arteries. FASEB J. 18:379–381 [DOI] [PubMed] [Google Scholar]
- 55. Werth N, et al. 2010. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS One 5:e11576 doi:10.1371/journal.pone.0011576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Worlitzsch D, et al. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yee Koh M, Spivak-Kroizman TR, Powis G. 2008. HIF-1 regulation: not so easy come, easy go. Trends Biochem. Sci. 33:526–534 [DOI] [PubMed] [Google Scholar]
- 58. Zhou J, Kohl R, Herr B, Frank R, Brune B. 2006. Calpain mediates a von Hippel-Lindau protein-independent destruction of hypoxia-inducible factor-1alpha. Mol. Biol. Cell 17:1549–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zinkernagel AS, Johnson RS, Nizet V. 2007. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. (Berl.) 85:1339–1346 [DOI] [PubMed] [Google Scholar]
- 60. Zinkernagel AS, Peyssonnaux C, Johnson RS, Nizet V. 2008. Pharmacologic augmentation of hypoxia-inducible factor-1alpha with mimosine boosts the bactericidal capacity of phagocytes. J. Infect. Dis. 197:214–217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.