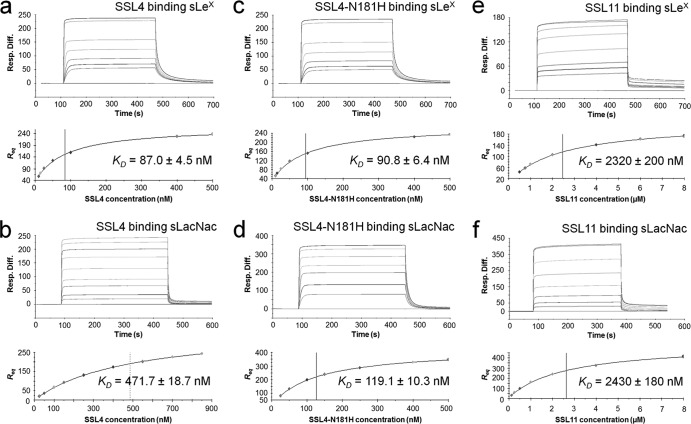
Fig 3.
Quantitative measure of SSL4t, SSL4t-N181H, and SSL11 binding to sLex and sLacNac. Binding responses (Resp.) at equilibrium (Req) are shown against the concentration and fitted to a steady-state affinity binding model to calculate an equilibrium affinity constant (KD). (a) sLex sensor chip binding and equilibrium binding analysis of 6.125 to 500 nM SSL4t in duplicate. The KD was calculated to be 87.0 ± 4.5 nM. (b) sLacNac sensor chip binding and equilibrium binding analysis of 25 to 850 nM SSL4t in duplicate. The KD was calculated to be 471.7 ± 18.7 nM. (c) sLex sensor chip binding and equilibrium binding analysis of 6.125 to 500 nM SSL4t-N181H in duplicate. The KD was calculated to be 90.8 ± 6.4 nM. (d) sLacNac sensor chip binding and equilibrium binding analysis of 25 to 500 nM SSL4t-N181H in duplicate. The KD was calculated to be 119.1 ± 10.3 nM. (e) sLex sensor chip binding and equilibrium binding analysis of 0.5 to 8 μM SSL11 in duplicate. The KD was calculated to be 2.32 ± 0.20 μM. (f) sLacNac sensor chip binding and equilibrium binding analysis of 0.125 to 8 μM SSL11 in duplicate. The KD was calculated to be 2.43 ± 0.02 μM. The plots shown are representative of two independent experiments, Rmax and χ2 values can be found in Table S1 in the supplemental material.