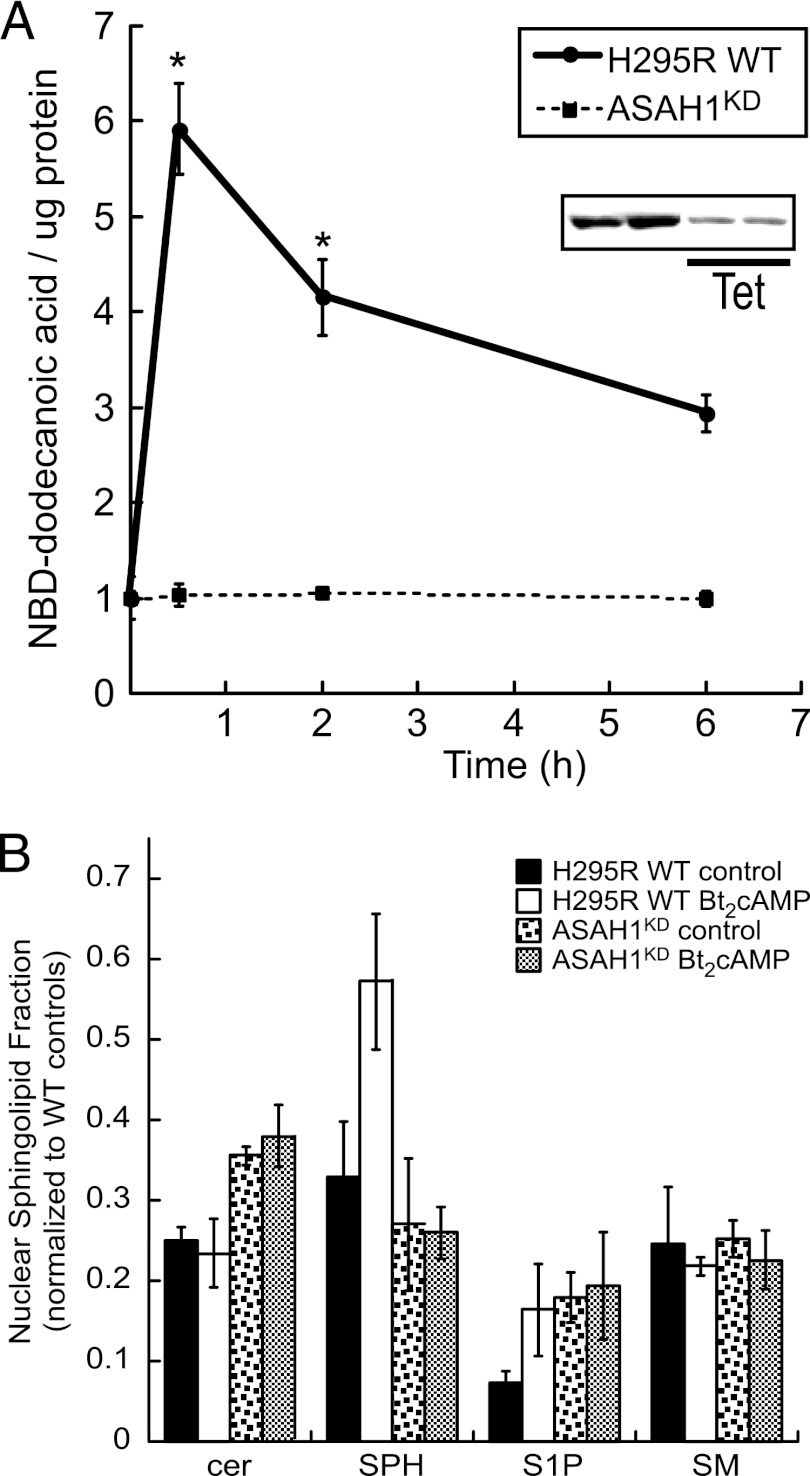
Fig 2.
ACTH/cAMP signaling promotes nuclear sphingolipid metabolism in an ASAH1-dependent manner. (A) H295R wild-type (WT) and ASAH1 knockdown (ASAH1KD) (pretreated for 96 h with 5 μg/ml tetracycline [Tet]) cells were treated for 30 min or 2 or 6 h with 0.4 mM Bt2cAMP, and nuclear lysates were isolated as described in Materials and Methods. H295R WT or ASAH1KD nuclear extracts (150 μl) were incubated with 2 μl 1 mM NBD-12-ceramide in acetate buffer (0.5 M, pH 4.5) at 37°C for 2 h. Reactions were stopped, and lipids were extracted and spotted on TLC plates. Plates were developed and visualized by fluorescent scanning. NBD-dodecanoic acid formation was quantified and normalized to the protein content of each sample. Data represent means ± standard errors of the means (SEMs) from 3 separate experiments, each performed in duplicate. (Inset) Representative Western blot of controls and Tet-treated H295RKD cells demonstrating decreased ASAH1 protein levels. *, statistically different from value for untreated control group (P < 0.05). (B) H295R WT or ASAH1KD (pretreated for 96 h with 5 μg/ml Tet) cells were subcultured into 100-mm dishes and treated with 0.4 mM Bt2cAMP for 30 min, and sphingosine (SPH), ceramide (cer), sphingosine-1-phosphate (S1P), and sphingomyelin (SM) concentrations in purified whole cells or nuclei were quantified by LC-ESI-MS-MS using appropriate standards and normalized to total cellular protein concentrations. Data are graphed as the means ± standard deviations of two experiments, each performed in duplicate, and are plotted as the nuclear fraction of each (nuclear/cellular) sphingolipid species with normalization to the amounts for wild-type untreated whole-cell sphingolipid.