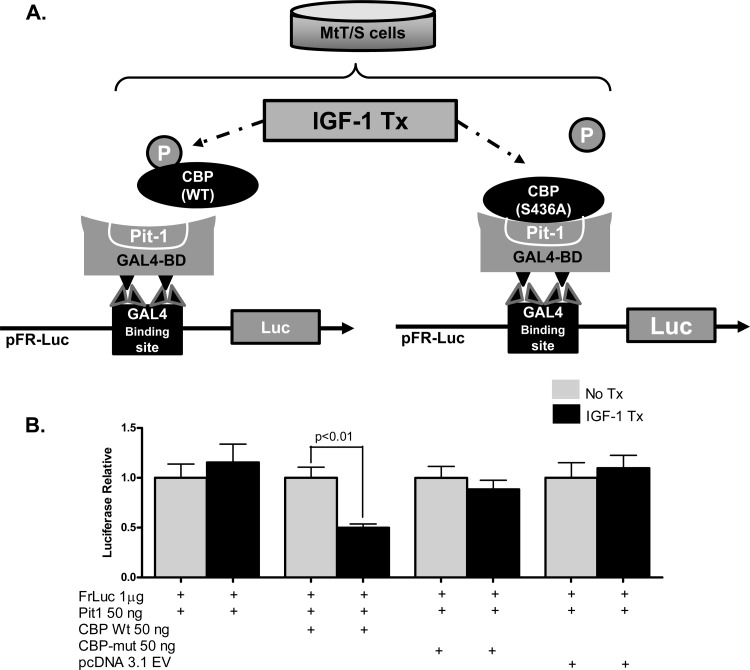
Fig 5.
IGF-1-mediated phosphorylation of CBP disrupts the Pit-1/CBP interaction. (A) Diagram of the experimental MtT/S cell transfection strategy used to determine the significance of CBP phosphorylation. Cotransfection of the pFR-Luc reporter vector containing GAL4 binding sites, wild-type (WT) Pit-1 inserted into a pFA-CMV vector containing the GAL4 binding domain (GAL4-BD) sites, and wild-type CBP inserted into a pcDNA3.1(−) vector demonstrated measureable luciferase expression. The interaction of Pit-1 and CBP is represented in the diagram. Previous data suggest that IGF-1R signaling leads to phosphorylation of CBP and disrupts the Pit-1/CBP complex; this would have led to decreased luciferase expression in these experiments. This mechanism of inhibition was tested by using a mutant CBP, CBP (S436A), which cannot be phosphorylated. (B) IGF-1 treatment of transfected cells with Pit-1 and WT CBP leads to a significant decrease in relative luciferase expression. Transfections using mutant CBP (S436A) show no change in luciferase expression, therefore demonstrating a loss of IGF-1 inhibition. No significant differences in luciferase expression were seen in cells treated with pcDNA3.1 EV, which served as a control. Shown are means + SEM.