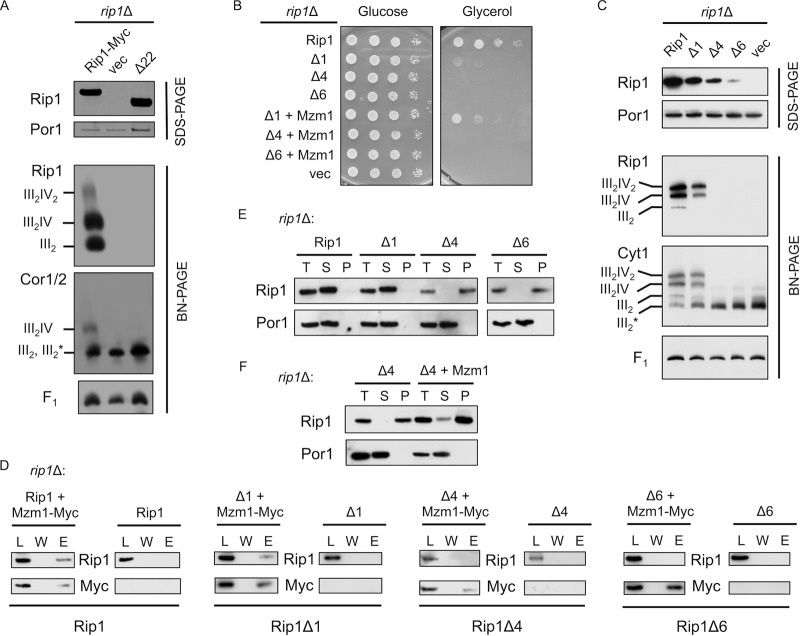
Fig 5.
The extreme C terminus of Rip1 is essential for its function and important for interaction with Mzm1. (A) SDS-PAGE and BN-PAGE (digitonin) immunoblots of isolated mitochondria from WT and rip1Δ cultures expressing YEp RIP1-Myc, vector control (vec), or rip1Δ22 plasmid. Note that on this BN gel the dimeric bc1 and late core intermediate comigrate, so the band designation includes both III2 and III2*, the latter of which denotes the late core intermediate formed during bc1 complex assembly. The component is clearly the late core intermediate in the vector control rip1Δ cells. (B) Growth assay at 37°C of rip1Δ cultures expressing YCp RIP1, vector control, or the Δ1, Δ4, or Δ6 Rip1 truncation and coexpressing YCp vector control or MZM1-Myc plasmid. (C) SDS-PAGE and BN-PAGE (digitonin) immunoblots of rip1Δ cultures expressing YCp RIP1, Δ1, Δ4, Δ6, or vector control plasmid. (D) Immunoprecipitation of solubilized mitochondria from rip1Δ cultures expressing the plasmids listed in panel C with or without coexpression of YCp MZM1-Myc, using anti-Myc antibody-conjugated agarose beads and showing the total load (L), the final wash (W), and the eluate (E). (E) Detergent-based protein aggregation assay and SDS-PAGE immunoblot of the total load (T), soluble fraction (S), and pellet fraction (P), as described for Fig. 2 except that cultures were grown at 30°C with mitochondria isolated from rip1Δ cultures expressing the same plasmids as in panel C. The exposure of the Rip1Δ6 blot was extended due to limited steady-state levels of this truncation. (F) Assay described in E for a rip1Δ culture expressing the Δ4 truncation, with and without coexpression of YCp MZM1-Myc.