Abstract
The P2X7 purinergic receptor is an ATP-gated cation channel with an emerging role in neoplasia. In this study we demonstrate that the human KG-1 cell line, a model of acute myelogenous leukaemia, expresses functional P2X7. RT-PCR and immunochemical techniques demonstrated the presence of P2X7 mRNA and protein respectively in KG-l cells, as well as in positive control multiple myeloma RPMI 8226 cells. Flow cytometric measurements demonstrated that ATP induced ethidium+ uptake into KG-l cells suspended in sucrose medium (EC50 of ∼3 μM), but not into cells in NaCl medium. In contrast, ATP induced ethidium+ uptake into RPMI 8226 cells suspended in either sucrose or NaCl medium (EC50 of ∼3 or ∼99 μM, respectively), as well as into RPMI 8226 cells in KCl medium (EC50 of ∼18 μM). BzATP and to a lesser extent ATPγS and αβ-methylene ATP, but not ADP or UTP, also induced ethidium+ uptake into KG-1 cells. ATP-induced ethidium+ uptake was completely impaired by the P2X7 antagonists, AZ10606120 and A-438079. ATP-induced ethidium+ uptake was also impaired by probenecid but not by carbenoxolone, both pannexin-1 antagonists. ATP induced YO-PRO-12+ and propidium2+ uptake into KG-1 cells. Finally, sequencing of full-length P2X7 cDNA identified several single nucleotide polymorphisms (SNPs) in KG-1 cells including H155Y, A348T, T357S and Q460R. RPMI 8226 cells contained A348T, A433V and H521Q SNPs. In conclusion, the KG-1 cell line expresses functional P2X7. This cell line may help elucidate the signalling pathways involved in P2X7-induced survival and invasiveness of myeloid leukaemic cells.
Keywords: Purinergic receptor, Extracellular ATP, Acute myelogenous leukaemia, Cation channel, Single nucleotide polymorphism
Introduction
The P2X7 purinergic receptor is a trimeric ATP-gated cation (Ca2+, Na+ and K+) channel present on various cell types [1]. Prolonged activation of P2X7 by extracellular ATP causes the uptake of organic cations, including ethidium+, and various downstream signalling events [1]. P2X7 plays important roles in inflammation and immunity, as well as in bone, epithelial and neuronal homeostasis [2, 3]. P2X7 also has an emerging role in neoplasia [4, 5]. Down-regulation of P2X7 may act as a tumour escape mechanism by preventing ATP-induced cell death [6]. Alternatively, P2X7 activation may promote ATP-induced tumour cell survival and growth [7, 8]. P2X7 activation may also facilitate tumour invasiveness and metastases [9] by inducing the release of matrix membrane metalloproteases [10] and cysteine cathepsins [11], and the shedding of cell adhesion molecules [12, 13]. Despite these findings however, the role of P2X7 in neoplasia remains poorly defined. This, in large part, is due to the lack of human tumour cell lines suitable for the study of P2X7, with many cell lines failing to express this receptor [14] despite the detection of P2X7 in various malignant tissues [15, 16]. Therefore, using molecular, immunochemical and pharmacological approaches we investigated whether the human KG-1 cell line, a commonly used model of acute myelogenous leukaemia [17], expresses functional P2X7 receptors.
Materials and methods
Materials
RPMI-1640 medium, GlutaMAX, YO-PRO-1 iodide and probenecid were from Invitrogen (Grand Island, NJ, USA). Foetal calf serum was from Lonza (Basel, Switzerland) or Bovogen Biologicals (East Kellior, Australia). Bovine serum albumin (BSA) and ethidium bromide were from Amresco (Solon, OH, USA). Nucleotides, propidium iodide and carbenoxolone were from Sigma Chemical Co (St Louis, MO, USA). AZ10606120 and A-438079 were from Tocris Bioscience (Ellisville, MO, USA).
Cell lines
KG-1 and RPMI 8226 cells (European Collection of Cell Cultures, Porton Down, UK) were maintained in complete culture medium (RPMI-1640 medium containing 2 mM GlutaMAX, and 20 % or 10 % fetal calf serum, respectively) at 37 °C/5 % CO2.
P2X7 expression
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RT-PCR of P2X7 mRNA was performed using Superscript III One-Step RT-PCR System Platinum Taq DNA polymerase (Invitrogen) as described [18]. Immunoblotting of whole cell lysates (the equivalent of 2 × 105 cells per lane) was performed using a rabbit anti-rat P2X7 polyclonal antibody (pAb; Alomone Labs, Jerusalem, Israel) as described [18]. A rabbit anti-actin pAb (Sigma Chemical Co) was used to confirm equal protein loading as described [18]. Mean fluorescence intensity (MFI) of cell-surface P2X7 expression was detected using an Alexa Fluor 647-conjugated murine anti-human P2X7 monoclonal antibody (mAb; clone L4) and flow cytometry as described [14].
Organic cation uptake measurements
Nucleotide-induced organic cation uptake into cells suspended in either sucrose (280 mM sucrose, 5 mM KCl, 10 mM N-methyl-d-glucamine, 5 mM glucose, 0.1 % BSA, 10 mM HEPES, pH 7.4), NaCl (145 mM NaCl, 5 mM KCl, 5 mM glucose, 0.1 % BSA, 10 mM HEPES, pH 7.4) or KCl (150 mM KCl, 5 mM glucose, 0.1 % BSA, 10 mM HEPES, pH 7.4) medium was determined using a fixed-time flow cytometric assay as described [14]. The fluorescence of the organic cations ethidium+, YO-PRO-12+ and propidium2+ were collected with band pass filters 575/26, 515/20 and 695/40, respectively.
cDNA sequencing
Total RNA was isolated as above. RT-PCR was performed using the MyTaq One-Step RT-PCR Kit (Bioline, Sydney, Australia) according to the manufacturer’s instructions using primer pairs (GeneWorks, Hindmarsh, Australia) specific for four overlapping regions of full-length P2X7 cDNA including parts of the untranslated 5′ and 3′ ends. The primer pairs (forward and reverse, respectively) were: (1) 5′-tggccctgtcaggaagagta-3′ and 5′-caccaggcagagacttcaca-3′; (2) 5′-ttgtaaaaagggatggatgga-3′ and 5′-aaatatgggagcgacagcag-3′; (3) 5′-tacatcggctcaaccctctc-3′ and 5′-gaacagctctgaggtggtga-3′; and (4) 5′-gtctggtgccagtgtggaa-3′ and 5′-actcccgacctcaggtgat-3′. The PCR cycling conditions were 45 °C for 20 min, 95 °C for 1 min, 40 cycles of 95 °C for 10 s, 63 °C (primer pair 1), 59 °C (primer pair 2), 62 °C (primer pair 3) or 61 °C (primer pair 4) for 10 s, and 72 °C for 30 s, and a final step of 72 °C for 5 min. Amplicons were ran on 2 % agarose gels, visualized by ethidium bromide staining, excised and purified using the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Purified amplicons were sequenced using the above primers with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA) and an Applied Biosystems 3130xl Genetic Analyzer. PCR cycling conditions for sequencing were 95 °C for 2 min, and then 25 cycles of 96 °C for 30 s, 55 °C for 15 s and 60 °C for 4 min.
Data presentation and statistics
Data are presented as mean ± SD. Differences between treatments were compared using either the unpaired Student’s t test for single comparisons to control samples or ANOVA for multiple comparisons (using Tukey’s post test) using Prism 5 (Mac OS X Version 5.0a; GraphPad Software, San Diego, CA, USA). Concentration response curves of log(agonist) vs. normalized response were fitted using the least squares (ordinary) fit method using Prism 5.
Results
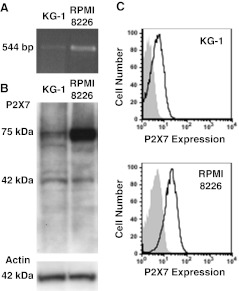
RT-PCR demonstrated P2X7 mRNA expression in KG-1 cells, as well as in positive control RPMI 8226 cells (Fig. 1a), a human multiple myeloma cell line which expresses high amounts of functional P2X7 [14]. Immunoblotting with an anti-P2X7 pAb revealed the presence of a major band at 75 kDa, the predicted size of glycosylated P2X7, in both cell types (Fig. 1b). A minor band at 42 kDa was also observed in both cell lines (Fig. 1b). The amount of P2X7 was lower in KG-1 cells compared to RPMI 8226 cells, despite equal protein loading as demonstrated using an anti-actin pAb (Fig. 1b). Finally, immunolabelling with an anti-P2X7 mAb and flow cytometric analysis demonstrated cell-surface P2X7 expression on KG-1 cells, which was significantly lower than that on RPMI 8226 cells (MFI of 3.3 ± 0.4 and 17.0 ± 3.2 respectively, n = 3, P < 0.01; Fig. 1c).
Fig. 1.
KG-1 cells express P2X7. a RNA isolated from KG-1 and RPMI 8226 cells was amplified by RT-PCR using primers to P2X7 and products examined by agarose gel electrophoresis. b Whole cell lysates from KG-1 and RPMI 8226 cells were separated by SDS-PAGE, transferred to nitrocellulose membrane and probed with anti-P2X7 (top panel) or anti-actin (bottom panel) pAb. c KG-1 and RPMI 8226 cells were labeled with Alexa Fluor 647-conjugated P2X7 (solid line) or isotype control (shaded) mAb, and the relative cell-surface P2X7 expression measured by flow cytometry. Representative results from (a,b) two or c three experiments shown
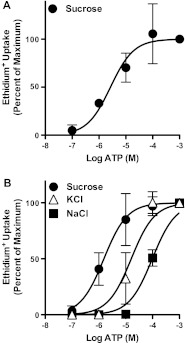
Preliminary experiments demonstrated that incubation of KG-1 cells suspended in NaCl medium with 1 mM ATP for up to 20 min failed to induce ethidium+ uptake into these cells, despite significant ethidium+ uptake into RPMI 8226 cells in NaCl medium after 5 min ATP incubation (results not shown). Therefore, ATP-induced ethidium+ uptake into KG-1 cells was next assessed in sucrose medium, which can facilitate P2X7-induced cation uptake in other cell types [19, 20]. A 20 min incubation with ATP induced significant ethidium+ uptake into KG-1 cells in a concentration–dependent manner, with a maximal uptake at 100 μM ATP and with an EC50 of 3.3 ± 1.3 μM (Fig. 2a). This EC50 value is typical of cation fluxes mediated by recombinant human P2X7 expressed in cell lines in sucrose medium [19]. Moreover, this EC50 value was similar to the EC50 value (2.7 ± 2.7 μM) for ATP-induced ethidium+ uptake into RPMI 8226 cells suspended in sucrose medium (Fig. 2b). In contrast, the EC50 values for ATP-induced ethidium+ uptake into RPMI 8226 cells suspended in either KCl or NaCl medium were higher (17.8 ± 8.2 or 99.3 ± 19.6 μM, respectively) (Fig. 2b). The latter EC50 value was similar to that previously observed for RPMI 8226 cells in NaCl medium [14].
Fig. 2.
ATP induces ethidium+ uptake into KG-1 cells. a KG-1 cells in sucrose medium or b RPMI 8226 cells in sucrose, KCl or NaCl medium were incubated with 25 μM ethidium+ in the presence of varying amounts of ATP (as indicated) at 37 °C for a 20 or b 5 min. Incubations were stopped by MgCl2 solution and centrifugation, and ethidium+ uptake measured by flow cytometry. Ethidium+ uptake is expressed as percent maximum response compared to 1 mM ATP for each corresponding medium; results are mean ± SD (a, n = 3; b, n = 9)
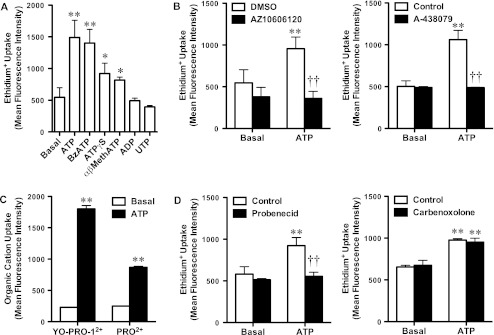
To determine whether the ATP-induced ethidium+ uptake into KG-1 cells was mediated by P2X7, cells suspended in sucrose medium were incubated in the absence or presence of ATP, the most potent P2X7 agonist BzATP, the partial P2X7 agonist ATPγS, or agonists of other P2 receptors. As above, 100 μM ATP induced a significant amount of ethidium+ uptake (Fig. 3a). Similarly, 100 μM BzATP, and to a lesser extent 100 μM ATPγS also induced significant ethidium+ uptake (Fig. 3a). Surprisingly, 100 μM αβ-methylene ATP, which is not a recognised P2X7 agonist, also induced a small but significant uptake of ethidium+ (Fig. 3a). In contrast, neither 100 μM ADP or UTP caused a significant amount of ethidium+ uptake into KG-1 cells (Fig. 3a). To confirm that this ATP-induced ethidium+ uptake was mediated by P2X7, KG-1 cells were pre-incubated in the absence or presence of the P2X7 antagonists, AZ10606120 or A-438079, and the relative amounts of ATP-induced ethidium+ uptake into KG-1 cells suspended in sucrose medium measured. Pre-incubation of cells with 100 nM AZ10606120 or 10 μM A-438079 completely inhibited 100 μM ATP-induced ethidium+ uptake (Fig. 3b). Neither antagonist significantly altered ethidium+ uptake in the absence of ATP (Fig. 3b).
Fig. 3.
P2X7 activation mediates organic cation uptake into KG-1 cells. a–d KG-1 cells were suspended in sucrose medium. a Cells were incubated with 25 μM ethidium+ in the absence (Basal) or presence of 100 μM nucleotide (as indicated; αβMethATP, αβ-methylene ATP) at 37 °C for 20 min. Cells were pre-incubated b with DMSO or 100 nM AZ10606120 (left panel), in the absence (Control) or presence of 10 μM A-438079 (right panel), d in the absence (Control) or presence of 2 mM probenecid (left panel), or in the absence (Control) or presence of 20 μM carbenoxolone (right panel) at 37 °C for 15 min. Cells were then incubated with b,d 25 μM ethidium+, c 1 μM YO-PRO-12+ or 25 μM propidium2+b–d in the absence (Basal) or presence of 100 μM ATP at 37 °C for 20 min. a–d Incubations were stopped by MgCl2 solution and centrifugation, and cation uptake measured by flow cytometry. Results are mean ± SD (n = 3–6); *P < 0.05 or **P < 0.01 compared to corresponding basal, ††P < 0.01 compared to corresponding ATP alone
Debate exists over the cut-off size of the P2X7 pore. Therefore, in addition to ethidium+ (314 Da) above, the effect of ATP incubation on YO-PRO-12+ (375 Da) and propidium2+ (415 Da) uptake into KG-1 cells suspended in sucrose medium was assessed. ATP induced significant uptake of both YO-PRO-12+ and propidium2+ (Fig. 3c). Debate also exists over the molecular identity of the P2X7 pore; in particular the hemichannel pannexin-1 may function as the P2X7 pore [21]. Therefore, KG-1 cells were pre-incubated in the absence or presence of the pannexin-1 antagonists, probenecid or carbenoxolone, and the relative amounts of ATP-induced ethidium+ uptake into KG-1 cells suspended in sucrose medium measured. Cells were pre-incubated with probenecid or carbenoxolone at concentrations reported to block pannexin-1 [21, 22]. Pre-incubation of cells with 2 mM probenecid significantly inhibited 100 μM ATP-induced ethidium+ uptake (Fig. 3d). In contrast, 20 μM carbenoxolone failed to alter 100 μM ATP-induced ethidium+ uptake (Fig. 3d). Neither antagonist significantly altered ethidium+ uptake in the absence of ATP (Fig. 3d).
The above data indicates that the low amount of P2X7 function in KG-1 cells, compared to RPMI 8226 cells, corresponds to low P2X7 expression. To determine if this low P2X7 function in KG-1 cells may be attributed to single nucleotide polymorphisms (SNPs) that alter P2X7 expression or function, full-length P2X7 cDNA from these cells was amplified by RT-PCR and sequenced. Full-length P2X7 cDNA from RPMI 8226 cells was also amplified and sequenced as a comparison. The p2x7 gene of KG-1 cells was heterozygous for six non-synonymous and three synonymous SNPs (Table 1). Of note, these cells contained the loss-of-function SNP, T357S [23], two gain-of-function SNPs, H155Y [24] and A348T [25, 26], and the Q460R SNP, which uniquely identifies the gain-of-function haplotype variant P2X7-4 [26]. The p2x7 gene of RPMI 8226 cells was homozygous for three non-synonymous and two synonymous SNPs (Table 1). Of note, these cells contained the A348T gain-of-function SNP, as well as the H521Q SNP, which reduces the sensitivity of the receptor to inhibition by extracellular Ca2+ [25]. Neither cell line encoded other P2X7 SNPs (reviewed in [1, 3]) including well-characterised SNPs such as R307Q, E496A and I568N (results not shown).
Table 1.
KG-1 and RPMI 826 cells contain several single nucleotide polymorphisms in the p2x7 gene
| dbSNP rs# Cluster IDa | Base changeb | Amino acid change | Effect on function | KG-1 | RPMI 8226 |
|---|---|---|---|---|---|
| rs28360448 | GTG>GTA | V154V | Synonymousc | Wild-type | Homozygous |
| rs208294 | CAT>TAT | H155Y | Gain | Heterozygous | Wild-type |
| rs73403850 | CTG>CCG | L320P | Unknown | Heterozygous | Wild-type |
| rs1718119 | GCT>ACT | A348T | Gain | Heterozygous | Homozygous |
| rs2230911 | ACT>AGT | T357S | Loss | Heterozygous | Wild-type |
| rs28360459 | GCG>GTG | A433V | Unknown | Wild-type | Homozygous |
| rs2230912 | CAG>CGG | Q460R | Possible loss | Heterozygous | Wild-type |
| rs3751144 | CCC>CCT | P474P | Synonymous | Heterozygous | Wild-type |
| rs2230913 | CAC>CAG | H521Q | Neutrald | Wild-type | Homozygous |
| rs3751142 | CTG>CTT | L534L | Synonymous | Heterozygous | Wild-type |
| rs77111027 | CCG>CTC | P582L | Unknown | Heterozygous | Wild-type |
| rs1621388 | CCG>CCA | P582P | Synonymous | Heterozygous | Homozygous |
ahttp://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=5027
bFull-length P2X7 cDNA from KG-1 and RPMI 8226 cells was amplified by RT-PCR and sequenced to identify potential base changes (single nucleotide polymorphisms)
cEach synonymous mutation is predicted to have neutral effect on function
dNeutral effect; however the mutant receptor displays reduced sensitivity to inhibition by extracellular Ca2+
Discussion
In the current study, we demonstrate that the acute myelogenous leukaemic KG-1 cell line expresses functional P2X7 receptors. First, RT-PCR, and immunoblotting and immunolabelling demonstrate the presence of P2X7 mRNA and protein in KG-1 cells, respectively. Second, ATP induced ethidium+ uptake into KG-1 cells with an EC50 of ∼3 μM, a value typical of cation fluxes mediated by recombinant human P2X7 expressed in cells in sucrose medium [19] or endogenous P2X7 in RPMI 8226 cells in sucrose medium (this study). Third, the most potent P2X7 agonist BzATP, as well as the partial P2X7 agonist ATPγS, induced ethidium+ into KG-1 cells. Fourth, the P2X7 antagonists, AZ10606120 and A-438079, prevented ATP-induced ethidium+ uptake into KG-1 cells.
The amount of functional P2X7 on KG-1 cells is relatively low compared to other cell lines. Previously, we have shown that 5 min ATP incubation of either RPMI 8226 cells, murine erythroleukaemia cells or murine RAW264.7 macrophages in NaCl medium results in significant ethidium+ uptake [14, 27]. In comparison, up to 20 min incubation with ATP failed to induce significant ethidium+ uptake into KG-1 cells in NaCl medium. This relatively low amount of functional P2X7 in KG-1 cells is most likely attributed to the low amounts of cell-surface P2X7, as observed when compared to RPMI 8226 cells. This low amount of cell-surface P2X7 corresponded with reduced P2X7 mRNA and total protein expression in KG-1 cells compared to RPMI 8226 cells. The RT-PCR assay used however was not quantitative nor did it include a house-keeping gene.
In addition to the low amount of P2X7 expression in KG-1 cells, these cells where heterozygous for the loss-of-function T357S SNP, which may further explain the reduced P2X7 function in KG-1 cells. The KG-1 cells were also heterozygous for the partial loss-of-function Q460R SNP, although the negative effect of this SNP on P2X7 function [26] has not been observed by others [25]. Moreover, in Caucasians (from which KG-1 cells originate), the Q460R SNP uniquely identifies a haplotype variant termed P2X7-4, which contains the gain-of-function SNPs, H155Y and A348T (but is wild-type at residue 357), and results in a net gain of function [26]. Nevertheless, given that the T357S SNP in heterozygous dosage reduces P2X7 function by at least 50 % in primary leukocytes from all subjects studied [23], it remains likely that the allele coding for S357 (and uniquely associated with haplotype variant P2X7-5 [26]) may have a dominant negative effect on other P2X7 alleles (including that containing P2X7-4) resulting in a net loss of function. KG-1 cells also contained two other non-synonymous SNPs, L320P and P582L, however their effect on P2X7 expression or function remains unknown. A study of rat P2X7, demonstrated that a P582G mutation fails to alter P2X7 channel or pore formation, although using truncated P2X7 variants this same study identified P582 as part of a pore-enabling region (551–582) which regulates cell-surface P2X7 expression and thereby P2X7 function [28]. As a result, these authors hypothesised that mutations within this region may prevent cell-surface P2X7 expression [28]. Thus, the possibility remains that P582L may reduce P2X7 function as a result of reduced cell-surface P2X7 expression. Alternatively, the L320P SNP, which resides within the ATP-binding site of P2X receptors [29], may result in an atypical pharmacology and contribute to the low P2X7 function in KG-1 cells. Atypical P2X7 pharmacology has also been observed in human osteosarcoma MG63 and SaOS2 cells [30]. Moreover, αβ-methylene ATP, which is not a recognised P2X7 agonist, induced a small but significant uptake of ethidium+ into KG-1 cells. Nucleotide preparations however can contain small amounts of contaminating ATP [31]. Thus, comparison of the ATP concentration response and agonist data suggests that as little as a 1 % contaminating ATP may have contributed to the αβ-methylene-induced ethidium+ uptake into KG-1 cells. Finally, we cannot exclude that KG-1 cells contain an alternate P2X7 isoform, rather than a SNP, that reduces P2X7 function as observed in various human epithelial cervical cancer cell lines [6].
The presence of p2x7 SNPs in cell lines is not unique to KG-1 cells. The human acute monocytic leukaemic THP-1 cell line is heterozygous for the loss-of-function E496A SNP [14, 32], while others have shown that human myelomonocytic leukaemic J6-1 cells contain the loss-of-function N187D SNP [33]. Moreover, the current study reveals that RPMI 8226 cells are homozygous for the gain-of-function A348T SNP, as well as A433V and H521Q. The presence of threonine at position 348 may account for the higher P2X7 function of RPMI 8226 cells compared to KG-1 cells. Although not formally demonstrated, A433V may also act as an additional gain-of-function SNP in these cells as the opposite amino acid exchange at position 76 of P2X7 (V76A) causes a loss of function [26]. Finally, others have shown that H521Q has no effect on ATP-induced ethidium+ uptake, but can reduce the sensitivity of P2X7 to inhibition by extracellular Ca2+ [25]. In this regard, maximal ATP-induced ethidium+ uptake into RPMI 8226 cells is similar in cells suspended in either NaCl medium containing Ca2+/Mg2+ or nominally free of Ca2+/Mg2+ [14].
The current study also demonstrates that P2X7 activation induces uptake of organic cations up to and including the size of propidium2+ (415 Da) into KG-1 cells. P2X7 activation induces ethidium+ (314 Da) but not propidium2+ uptake into human lymphocytes [34], dendritic cells [35] and THP-1 cells [18]. In contrast, P2X7 activation induces uptake of propidium2+ into rat bone nodule cells [36] and P2X7-transfected HEK-293 cells [37]. Reasons for differences between these cells, especially between the myeloid cell types (KG-1, dendritic and THP-1 cells) remain unknown. These differences may be due to technical reasons: both of the myeloid cell studies that failed to show P2X7-induced propidium2+ uptake were conducted with cells suspended in KCl medium, while P2X7-induced propidium2+ uptake into KG-1 cells was conducted with cells suspended in sucrose medium. Alternatively, the observed differences in P2X7-induced propidium2+ uptake between different cell types may reflect differences in the molecular identity of the P2X7 pore or in the propensity of P2X7 to dilatate. Attempts to characterize the molecular identity of the P2X7 pore in KG-1 cells using pannexin-1 antagonists suggest that the P2X7 pore in KG-1 cells is not pannexin-1 or that pannexin-1 is dispensable for P2X7-induced pore formation. ATP-induced ethidium+ uptake was completely blocked by probenecid but unaffected by carbenoxolone. Although probenecid can block pannexin-1 [22], it has long been recognised that probenecid blocks other molecules within cells [38]. Thus, the possibility remains that the P2X7 pore may be a molecule other than pannexin-1. Alternatively, probenecid may directly block P2X7 to prevent pore formation. Regardless, the lack of evidence for pannexin-1 as the P2X7 pore in KG-1 cells is consistent with studies using cells from pannexin-1 knockout mice, which are permeable to organic cations following ATP incubation [39].
P2X7 has been reported in various primary lymphoid and myeloid leukaemias in humans [40–44], however the pathophysiological significance of P2X7 in these malignant cell types remains unknown. Evidence indicates that the relative P2X7 expression and/or function directly correlates with the progression of chronic lymphocytic leukaemia [40] or acute myelogenous leukaemia [44] suggesting a role for increased P2X7 activity in promoting these leukaemias. Studies confirming these observations however are lacking. Nevertheless, the detection of functional P2X7 in KG-1 cells indicates that this cell line may provide a model to study the role of P2X7 in acute myelogenous leukaemia. For example, this cell line may be useful in determining if ATP induces death or survival in acute myelogenous leukaemic cells, and identifying the signalling pathways involved in either of these processes. Alternatively, given that KG-1 cell migration can be studied in vitro [45], and the potential role of P2X7 in facilitating tumour invasiveness and metastases [5], the KG-1 cell line might provide a useful model to further elucidate the role of P2X7 and downstream signalling pathways in this process.
Acknowledgments
This work was kindly supported by Cure Cancer Australia and the University of Wollongong. We gratefully acknowledged helpful advice from Marie Ranson, Mark Dowton and Simon Cook (all University of Wollongong), and excellent technical assistance by Margaret Phillips (University of Wollongong) and the staff of the Illawarra Health and Medical Research Institute. The authors have no conflicts of interest.
References
- 1.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321–332. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 2.Lenertz LY, Gavala ML, Zhu Y, Bertics PJ. Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions. Immunol Res. 2011;50:22–38. doi: 10.1007/s12026-011-8203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sluyter R, Stokes L. Significance of P2X7 receptor variants to human health and disease. Recent Pat DNA Gene Seq. 2011;5:41–54. doi: 10.2174/187221511794839219. [DOI] [PubMed] [Google Scholar]
- 4.Gorodeski GI. P2X7-mediated chemoprevention of epithelial cancers. Expert Opin Ther Targets. 2009;13:1313–1332. doi: 10.1517/14728220903277249. [DOI] [PubMed] [Google Scholar]
- 5.Roger S, Pelegrin P. P2X7 receptor antagonism in the treatment of cancers. Expert Opin Investig Drugs. 2011;20:875–880. doi: 10.1517/13543784.2011.583918. [DOI] [PubMed] [Google Scholar]
- 6.Feng YH, Li X, Zeng R, Gorodeski GI. Endogenously expressed truncated P2X7 receptor lacking the C-terminus is preferentially upregulated in epithelial cancer cells and fails to mediate ligand-induced pore formation and apoptosis. Nucleosides Nucleotides Nucleic Acids. 2006;25:1271–1276. doi: 10.1080/15257770600890921. [DOI] [PubMed] [Google Scholar]
- 7.Virgilio F, Ferrari D, Adinolfi E. P2X7: a growth-promoting receptor—implications for cancer. Purinergic Sig. 2009;5:251–256. doi: 10.1007/s11302-009-9145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F (2012) Expression of the P2X7 receptor increases in vivo tumor growth. Cancer Res. doi:10.1158/0008-5472.CAN-11-1947 [DOI] [PubMed]
- 9.Ren S, Zhang Y, Wang Y, Lui Y, Wei W, Huang X, Mao W, Zuo Y. Targeting P2X7 receptor inhibits the metastasis of murine P388D1 lymphoid neoplasm cells to lymph nodes. Cell Biol Int. 2010;34:1205–1211. doi: 10.1042/CBI20090428. [DOI] [PubMed] [Google Scholar]
- 10.Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107:4946–4953. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- 11.Jelassi B, Chantôme A, Alcaraz-Pérez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A, Roger S. P2X7 receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30:2108–2122. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson GP, Snook MB, Thurlow PJ, Wiley JS. Extracellular ATP causes of loss of L-selectin from human lymphocytes via occupancy of P2Z purinocepters. J Cell Physiol. 1996;166:637–642. doi: 10.1002/(SICI)1097-4652(199603)166:3<637::AID-JCP19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Ren S, Zhang L, Jin H, Sun J, Zuo Y (2011) Extracellular ATP induces CD44 shedding from macrophage-like P388D1 cells via the P2X7 receptor. Hematol Oncol. doi:10.1002/hon.1008 [DOI] [PubMed]
- 14.Farrell AW, Gadeock S, Pupovac A, Wang B, Jalilian I, Ranson M, Sluyter R. P2X7 receptor activation induces cell death and CD23 shedding in human RPMI 8226 multiple myeloma cells. Biochim Biophys Acta. 2010;1800:1173–1182. doi: 10.1016/j.bbagen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Deli T, Csernoch L. Extracellular ATP and cancer—an overview with special reference to P2 purinergic receptors. Pathol Oncol Res. 2008;14:219–231. doi: 10.1007/s12253-008-9071-7. [DOI] [PubMed] [Google Scholar]
- 16.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Koeffler HP, Golde DW. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978;200:1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- 18.Gadeock S, Tran JN, Georgiou JG, Jalilian I, Taylor RM, Wiley JS, Sluyter R. TGF-β1 prevents up-regulation of the P2X7 receptor by IFN-γ and LPS in leukemic THP-1 monocytes. Biochim Biophys Acta. 2010;1798:2058–2066. doi: 10.1016/j.bbamem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Michel AD, Chessell IP, Humphrey PP. Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:102–109. doi: 10.1007/PL00005328. [DOI] [PubMed] [Google Scholar]
- 20.Jursik C, Sluyter R, Georgiou JG, Fuller SJ, Wiley JS, Gu BJ. A quantitative method for routine measurement of cell surface P2X7 receptor function in leucocyte subsets by two-colour time-resolved flow cytometry. J Immunol Methods. 2007;325:67–77. doi: 10.1016/j.jim.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverman WR, Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, Britton WJ, Petrou S, Wiley JS. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and function impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006;281:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- 24.Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A, Agostini P, Cuneo A, Castoldi G, Baricordi OR, Virgilio F. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005;175:82–89. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- 25.Roger S, Mei ZZ, Baldwin JM, Dong L, Bradley H, Baldwin SA, Surprenant A, Jiang LH. Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X7 receptor functions. J Psychiatr Res. 2010;44:347–355. doi: 10.1016/j.jpsychires.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ, Wiley JS. Two haplotypes of the P2X7 receptor containing the Ala-348 to Thr polymorphism exhibit gain-of-function effect and enhanced interleukin-1β secretion. FASEB J. 2010;24:2916–2927. doi: 10.1096/fj.09-150862. [DOI] [PubMed] [Google Scholar]
- 27.Constantinescu P, Wang B, Kovacevic K, Jalilian I, Bosman GJ, Wiley JS, Sluyter R. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim Biophys Acta. 2010;1798:1797–1804. doi: 10.1016/j.bbamem.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Smart ML, Gu B, Panchel RG, Wiley JS, Cromer B, Williams DA, Petrou S. P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J Biol Chem. 2003;278:8853–8860. doi: 10.1074/jbc.M211094200. [DOI] [PubMed] [Google Scholar]
- 29.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alqallaf SM, Evans BAJ, Kidd EJ. Atypical P2X7 receptor pharmacology in two human ostoblast-like cell lines. Br J Pharmacol. 2009;156:1124–1135. doi: 10.1111/j.1476-5381.2009.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pojoga LH, Haghiac ML, Moose JE, Hilderman RH. Determination of ATP impurity in adenine dinucleotides. Nucleosides Nucleotides Nucleic Acids. 2004;23:581–598. doi: 10.1081/NCN-120030716. [DOI] [PubMed] [Google Scholar]
- 32.Sun C, Chu J, Singh S, Salter RD. Identification and characterization of a novel variant of the human P2X7 receptor resulting in gain of function. Purinergic Sig. 2010;6:31–45. doi: 10.1007/s11302-009-9168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong JH, Zheng GG, Ma YY, Zhang HY, Nie K, Lin YM, Wu KF. The hyposensitive N187D P2X7 mutant promotes malignant progression in nude mice. J Biol Chem. 2010;285:36179–36187. doi: 10.1074/jbc.M110.128488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiley JS, Chen R, Jamieson GP. The ATP4- receptor-operated channel P2Z of human lymphocytes allows Ba2+ and ethidium+ uptake—inhibition of fluxes by suramin. Arch Biochem Biophys. 1993;305:54–60. doi: 10.1006/abbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- 35.Sluyter R, Wiley JS. Extracellular adenosine 5′-triphosphate induces a loss of CD23 from human dendritic cells via activation of P2X7 receptors. Int Immunol. 2002;14:1415–1421. doi: 10.1093/intimm/dxf111. [DOI] [PubMed] [Google Scholar]
- 36.Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, Dixon SJ. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol. 2008;181:859–871. doi: 10.1083/jcb.200708037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milius D, Gröger-Arndt H, Stanchev D, Lange-Dohna C, Rossner S, Sperlagh B, Wirkner K, Illes P. Oxygen/glucose deprivation increases the integration of recombinant P2X7 receptors into the plasma membrane of HEK293 cells. Toxicology. 2007;238:60–69. doi: 10.1016/j.tox.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 38.Virgilio F, Steinberg TH, Silverstein SC. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990;11:57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- 39.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol Methods. 2011;186:6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- 40.Adinolfi E, Melchiorni L, Falzoni S, Chiozzi P, Morelli A, Tieghi A, Cuneo A, Castoldi G, Virgilio F, Baricordi OR. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99:706–708. doi: 10.1182/blood.V99.2.706. [DOI] [PubMed] [Google Scholar]
- 41.Chong JH, Zheng GG, Zhu XF, Guo Y, Wang L, Ma CH, Liu SY, Xu LL, Lin YM, Wu KF. Abnormal expression of P2X family receptors in Chinese pediatric acute leukemias. Biochem Biophys Res Commun. 2010;391:498–504. doi: 10.1016/j.bbrc.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 42.Shemon AN, Sluyter R, Wiley JS. Rottlerin inhibits P2X7 receptor stimulated phospholipase D activity in chronic lymphocytic leukaemia B-lymphocytes. Immunol Cell Biol. 2007;85:68–72. doi: 10.1038/sj.icb.7100005. [DOI] [PubMed] [Google Scholar]
- 43.Wiley JS, Dubyak GR. Extracellular adenosine triphosphate increases cation permeability of chronic lymphocytic leukemia lymphocytes. Blood. 1989;73:1316–1323. [PubMed] [Google Scholar]
- 44.Zhang XJ, Zheng GG, Ma XT, Yang YH, Li G, Rao Q, Nie K, Wu KF. Expression of P2X7 in human hematopoietic cell lines and leukemia patients. Leuk Res. 2004;28:1313–1322. doi: 10.1016/j.leukres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Janiak M, Hashmi HR, Janowska-Wieczorek A. Use of the Matrigel-based assay to measure the invasiveness of leukemic cells. Exp Hematol. 1994;22:559–565. [PubMed] [Google Scholar]