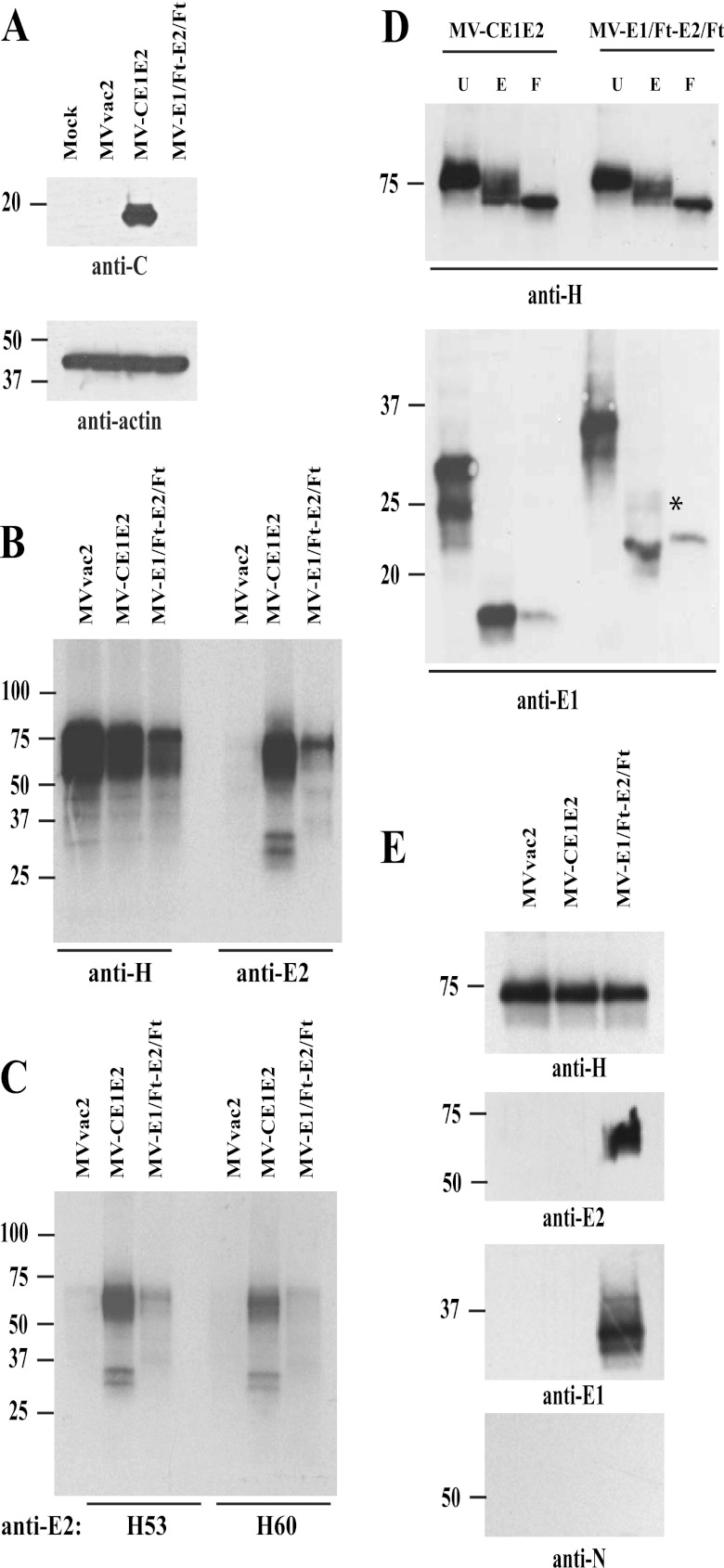
Fig 2.
Biochemical characterization of HCV proteins expressed by vectored MVs. (A) Expression of HCV core protein by vectored MV-CE1E2. Extracts from mock-, MVvac2-, MV-CE1E2-, or MV-E1/Ft-E2/Ft-infected cells were analyzed by immunoblotting. The reactivity of each antibody is indicated below each blot. (B) Immunoprecipitation of proteins produced by cells infected with MVvac2, MV-CE1E2, or MV-E1/Ft-E2/Ft at an MOI of 0.5. Proteins were labeled with [35S]methionine 24 h postinfection and precipitated with the antibody indicated below the lanes. The positions of molecular mass standards are indicated on the left. (C) Immunoprecipitation of HCV envelope proteins using conformation-dependent monoclonal antibodies H53 and H60, as indicated below the lanes. (D) Glycosidase treatment. MV H and HCV E1 complexes immunoprecipitated from cells infected with MV-CE1E2 or MV-E1/Ft-E2/Ft were treated with endoglycosidase H (E) or PNGase-F (F) or left untreated (U). MV H (top panel) and HCV E1 glycoproteins (botttom panel) were detected by immunoblotting using specific antibodies. The asterisk beside E1 indicates an endoglycosidase H-resistant form. (E) Surface biotinylation analysis. Cells were infected with MVvac2, MV-CE1E2, or MV-E1/Ft-E2/Ft at an MOI of 0.1. Forty-eight hours later, cells were labeled with biotin and lysed. Biotin-labeled proteins were pulled down with streptavidin-agarose and analyzed by immunoblotting. Antibodies used for specific detection are indicated at the bottom.