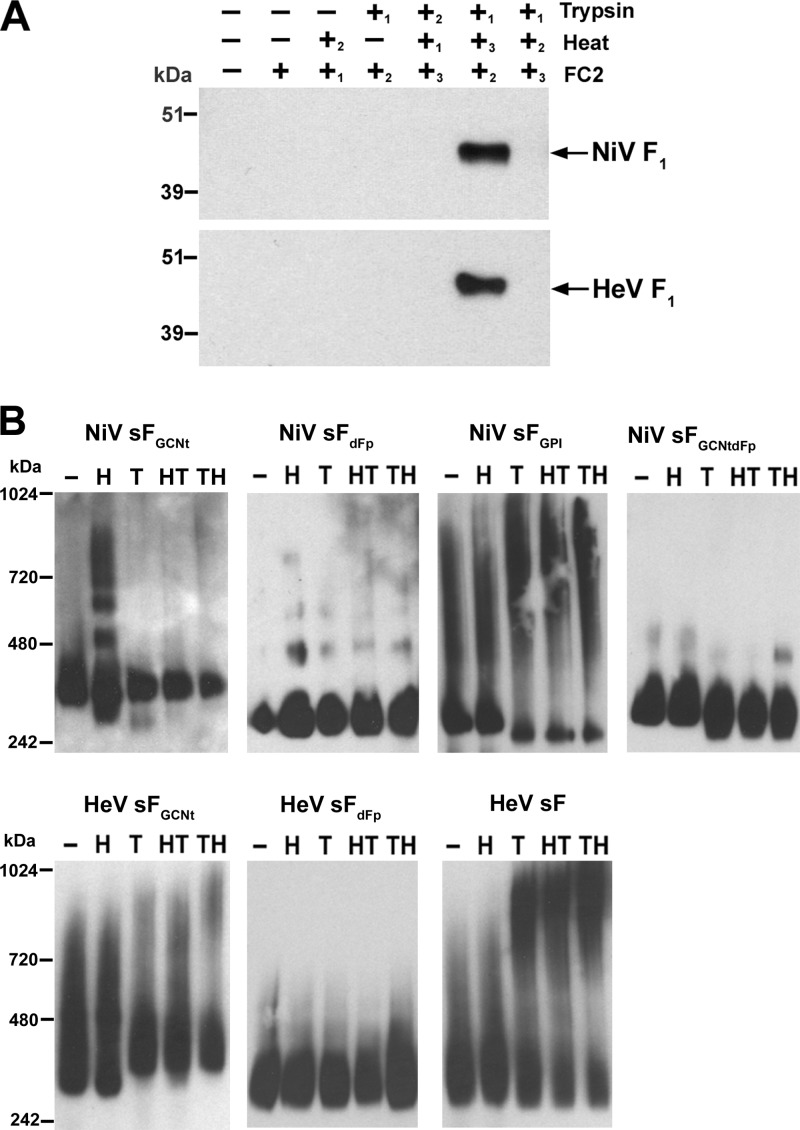
Fig 7.
In vitro processing of purified sFGCNt from a pre- to postfusion conformation and analysis of conformational changes. (A) Capture of a conformational intermediate during in vitro processing of sF by biotinylated FC2 heptad peptide. The NiV (top) and HeV (bottom) sFGCNt were untreated (−) or treated (+) with combinations of heat (50°C, 15 min), trypsin, and FC2 heptad peptide in a sequence indicated by the numbers (1 followed by 2 followed by 3). The sF-FC2 complexes were precipitated with avidin agarose and boiled in sample buffer. The precipitated proteins were resolved by 4 to 12% BT SDS-PAGE, followed by Western blotting. Blots were probed with rabbit anti-HeV F1 to probe for F. (B) Mobility shift in native PAGE of the different sF glycoprotein samples that were alternatively treated as indicated: untreated (−) or treated using heat only (H), trypsin only (T), heat followed by trypsin (HT), or trypsin followed by heat (TH). The treated proteins were resolved by 3 to 12% native PAGE, followed by Western blotting using anti-S-peptide-tag antibody to detect sF.