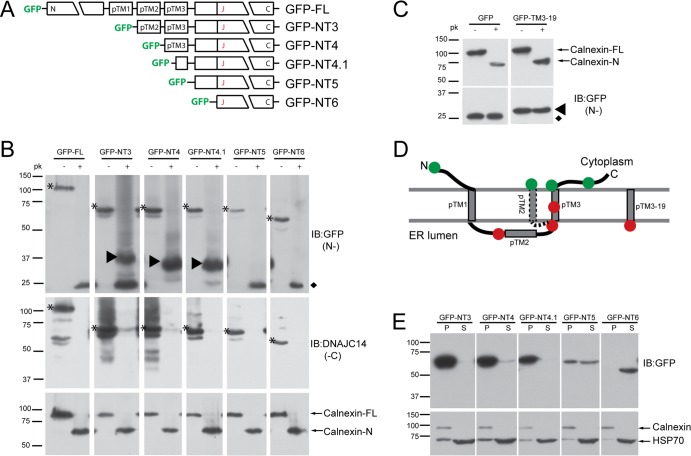
Fig 1.
Studies of DNAJC14 topology. (A) Schematic of DNAJC14-FL and pTrip-GFP mutant constructs fused in frame to the C terminus of GFP. The three individual putative TMs (pTMs) are indicated. (B) SW13 cells transduced with the indicated DNAJC14 mutant constructs were permeabilized with 50 μg/ml digitonin, scraped, and resuspended in buffer for treatment with 10 μg/ml proteinase K (pk) or no treatment. Proteins were TCA precipitated and analyzed by Western blotting with the antibodies indicated (IB). Top panels assessed N-terminal protection (N-), while the middle panels assessed C-terminal protection (-C). Arrowheads in the top panel indicate protected N-terminal GFP-containing proteins. Asterisks indicate the undigested DNAJC14 mutant constructs. The diamond indicates the protease-resistant GFP domain released after exposure to proteinase K. Calnexin (bottom panels) serves as a control for proteinase K digestion. (C) SW13 cells expressing GFP or GFP with 19 amino acids from pTM3 fused to the C terminus were treated and analyzed as in panel B. The protease-resistant GFP domain released by proteinase K is indicated by the diamond, and the protected larger species containing the TM sequence is indicated by the arrowhead. (D) Summary of proteinase K digestion results. The N-terminal GFP tag location is represented by a circle; green indicates that the GFP was not protected, while red indicates protection from proteinase K digestion. The alternative topology of pTM2 is indicated (dashed line). (E) Subcellular fractionation of DNAJC14 mutant constructs. Cells were disrupted by hypotonic buffer, and postnuclear lysates were separated by centrifugation into a crude membrane fraction (P) and a cytosolic fraction (S), which were analyzed by Western blotting.