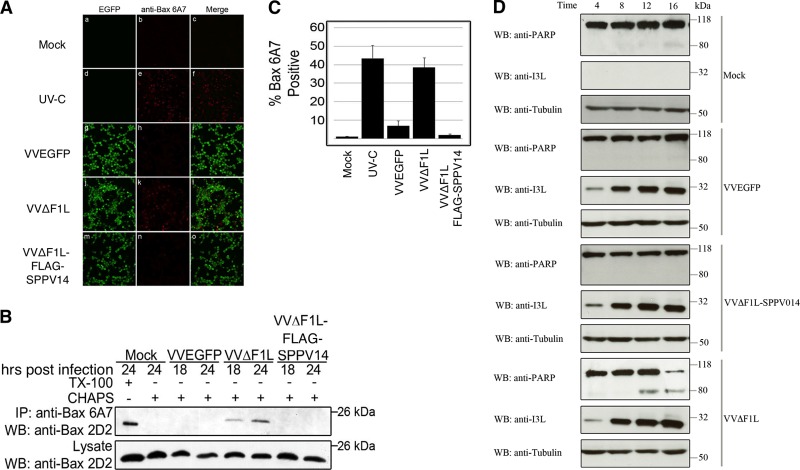
Fig 5.
SPPV14 inhibits Bax activation during vaccinia virus infection. (A) HeLa cells were infected with VVEGFP, VVΔF1L, or VVΔF1L-FLAG-SPPV14 and incubated for 24 h. Infected cells or UV-C-irradiated HeLa cells were fixed and stained with the conformation-specific anti-Bax antibody 6A7, which specifically recognizes activated Bax (14, 31). (B) HeLa cells infected with VVEGFP, VVΔF1L, or VVΔF1L-FLAG-SPPV14 were lysed with Triton X-100 or CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}-based buffers and immunoprecipitated (IP) using the conformation-specific anti-Bax antibody 6A7. The immunoprecipitants were subjected to SDS-PAGE and blotted with the anti-Bax antibody 2D2, an antibody that recognizes all forms of Bax in immunoblots. (C) SPPV14 inhibits Bax activation induced by VVΔF1L. HeLa cells infected with VVEGFP, VVΔF1L, or VVΔF1L-FLAG-SPPV14 were incubated for 24 h and fixed, and Bax activation was measured by staining with anti-Bax 6A7 antibody (31) that specifically recognizes activated Bax. The data were quantified by counting 200 cells per experiment; means and SD of three replicate experiments are shown. UV-C, positive-control UV-irradiated HeLa cells. (D) SPPV14 inhibits PARP cleavage induced by VVΔF1L. Jurkat cells were mock infected or infected with VVEGFP, VVΔF1L-SPPV14, or VVΔF1L at an MOI of 5. Samples were collected 4, 8, 12, and 16 h postinfection, and the cells were lysed in SDS-PAGE sample buffer containing 8 M urea. Samples were subjected to SDS-PAGE and immunoblotted for PARP to determine the trigger of apoptosis; β-tubulin was used as a loading control and I3L as a sign of infection.