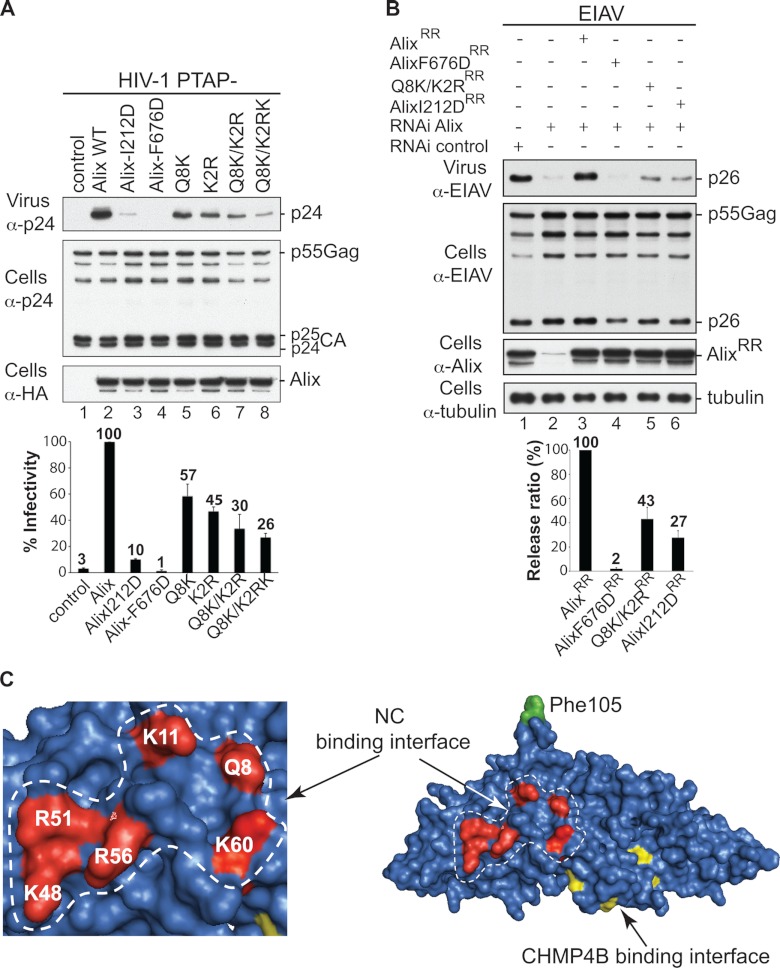
Fig 3.
Alix Bro1 interface involved in NC binding plays a critical role in virus release. (A) The Alix Bro1 mutants with a compromised NC binding interface show a decreased ability to stimulate the release of the HIV-1 PTAP− mutant. 293T cells were transfected with HIV-1 PTAP− proviral DNA alone (lane 1), with WT HA-tagged Alix (lane 2), or with the indicated Alix mutants (lanes 3 to 8). Cells and viruses were collected 24 h posttransfection, and their protein content was analyzed by SDS-PAGE and Western blotting using the indicated antibodies. Release of infectious viral particles was quantified using HeLa TZM-bl assays from five independent experiments and expressed relative to WT Alix (lane 2). Error bars represent standard deviations (SD). (B) An intact NC binding interface is required for Alix function in EIAV release. 293T cells were transfected twice with Alix RNAi oligonucleotides at 36-h intervals. At the second transfection, cells were cotransfected with EIAV provirus alone (lanes 1 and 2), with an RNAi-resistant (RR) version of Alix WT (lane 3), or with the indicated mutants (lanes 4 to 6). Cells and viruses were collected 24 h posttransfection, and their protein content was analyzed by SDS-PAGE and Western blotting using the indicated antibodies. Release ratios were calculated as described in Materials and Methods from 3 independent experiments and expressed relative to WT Alix (lane 3), ±SD. (C) Alix Bro1 residues engaged in NC interaction define a new functional interface. Residues Q8, K11, K48, R51, R56, and K60 placed in the Alix Bro1 domain crystal structure (2OEW) cluster within a positively charged exposed surface (shown in red) on one side of the Bro1 domain. For reference, the Phe105 residue (required for Alix function in HIV-1 release [36]) and the CHMP4B binding interface (27) are shown in green and yellow, respectively.