Fig 8.
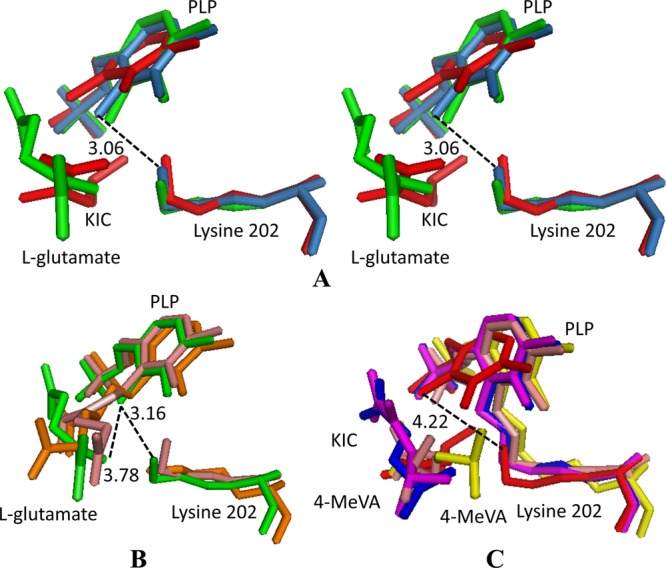
Superposition of the PLP, substrate, and lysine residues from the DrBCAT, hBCATm, hBCATc, HB8BCAT, and eBCAT. (A) Stereo view of a conformational comparison of the PLP, substrate, and Lys (PLP-substrate-Lys) in native DrBCAT (blue-gray), DrBCAT–l-glutamate (green), and DrBCAT-KIC (red) complexes. (B) Structural comparison of PLP-substrate-Lys of DrBCAT–l-glutamate (green), eBCAT–l-glutamate (salmon), and HB8BCAT–l-glutamate (orange, on the bottom). (C) Structural comparison of PLP-substrate-Lys of DrBCAT-KIC (red), hBCATm-C315A-C318A-KIC (magenta), eBCAT–4-MeVA (orange), oxidized hBCATc–4-MeVA (blue), and HB8BCAT–4-MeVA (yellow). The Lys202 is noncovalently linked to the PLP with distances of 3.06, 3.16, and 4.22 Å between C4A of PLP and NZ of Lys202 for native DrBCAT, DrBCAT–l-glutamate, and DrBCAT-KIC, respectively.
