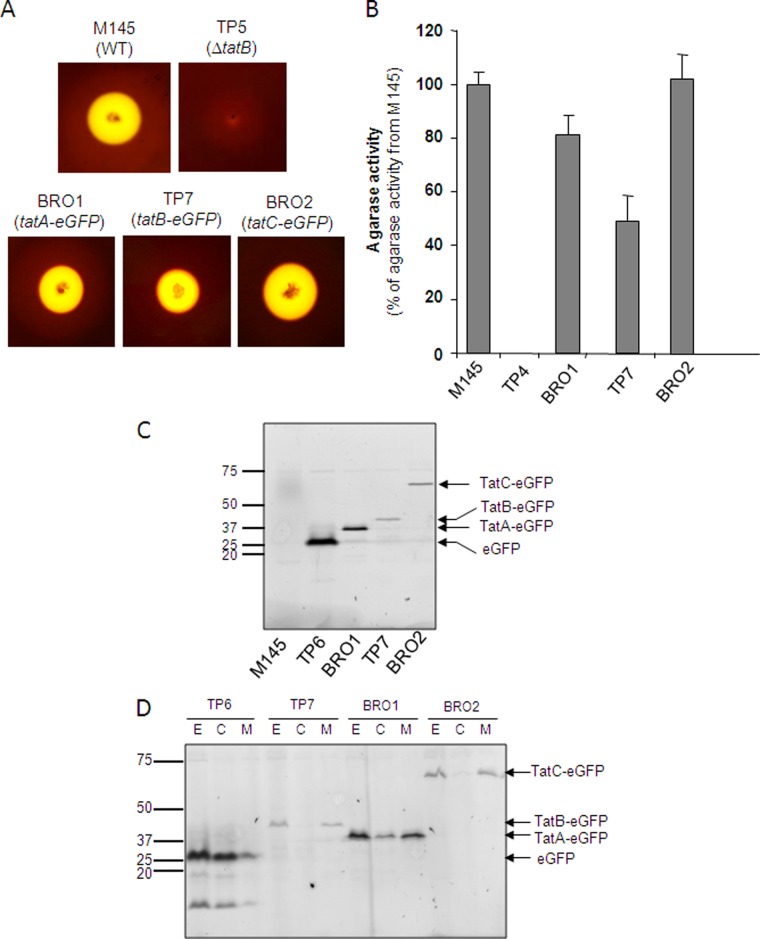
Fig 1.
Fusions of eGFP to the TatA, TatB, and TatC proteins of S. coelicolor are stable and do not abolish Tat transport activity. (A, B) Semiquantitative analysis of extracellular agarase activity mediated by S. coelicolor strains. The indicated strains were cultured on MM medium for 5 days at 30°C, after which they were stained with Lugol's solution. In panel A, zones of clearing around colonies after staining with Lugol's solution are shown, while panel B, shows relative agarase activity, which was estimated as described previously (51). The error bars represent the standard error of the mean; n = 6 to 9. (C, D) S. coelicolor strains M145, TP6 (M145 ΔtatB ϕC31 tatBp-tatBstop-eGFP), BRO1 (M145 tatA::tatA-eGFP), TP7 (M145 ΔtatB ϕC31 tatBp-tatB-eGFP), and BRO2 (M145 tatC::tatC-eGFP) were cultured aerobically for 24 h at 30°C in a 1:1 mixture of TSB and YEME media. Cell extracts (formed by sonication of hyphal suspension) (panel C) and subcellular fractions fractionated from extract (lanes E) into cytoplasmic (lanes C), and membrane (lanes M) fractions (panel D) were separated by SDS-PAGE (12% acrylamide; samples were not boiled prior to separation), and fluorescent proteins were analyzed by phosphorimaging. Twenty micrograms of total protein was loaded into each lane. The values to the left of panels C and D are molecular sizes in kilodaltons.