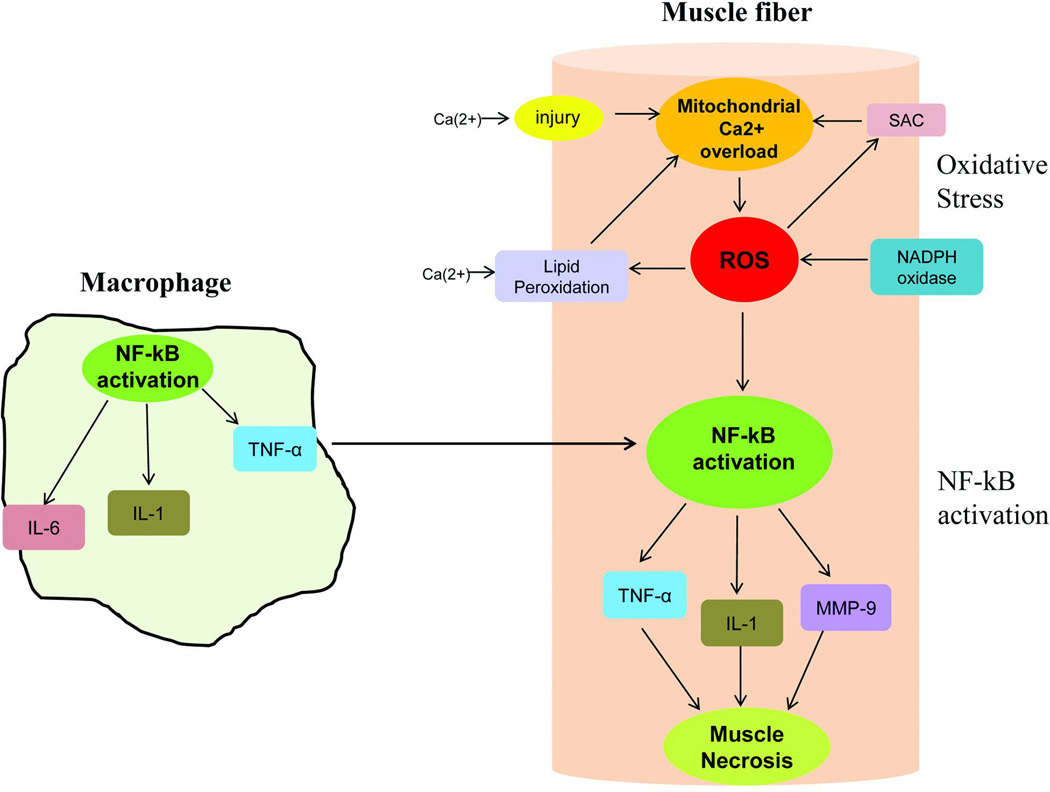
Figure 1.
Oxidative and NF-kB pathways are highly interactive in DMD muscle collaborating to produce muscle fiber necrosis. Macrophages generate inflammatory cytokines and reactive oxidative species (ROS) are the direct consequence of dystrophin deficiency and membrane instability. The illustration shows a macrophage with activated NF-κB leading to secretion of pro-inflammatory molecules, TNF-α, IL-1 & IL-6. A dystrophic muscle fiber shows reactive oxygen species generated by multiple insults to the muscle fiber: 1) Ca2+ influx through muscle membrane related to membrane fragility leads to Ca2+ overload in the mitochondria and energy deficiency; 2) increased NADPH oxidase activity known to accompany dystrophin deficiency adds to ROS generation which in turn stimulates stretch-activated channels (SAC) resulting in Ca2+ entry into muscle fiber further contributing to mitochondrial dysfunction; 3) ROS exacerbates the dystrophic pathology by peroxidation of the lipid bilayer of the muscle membrane. ROS provokes the NF-κB signaling pathway leading to production of TNF-α and other cytokines. Moreover, TNF-α produced by activated macrophages enhances NF-κB activation in the muscle.