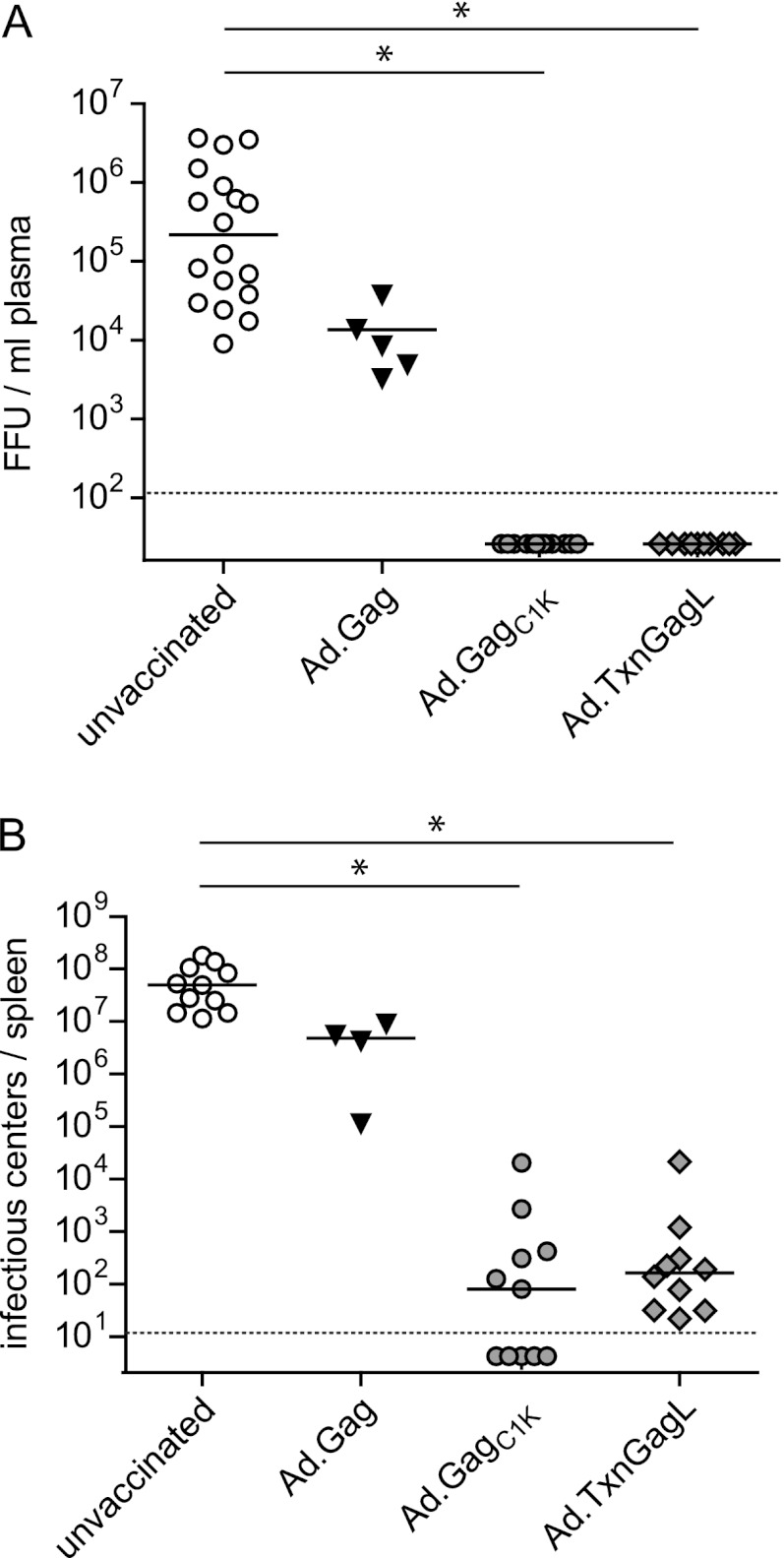
Fig 3.
Protection from high-dose FV challenge infection. CB6F1 mice were immunized twice in a 3-week interval using 109 vp of adenoviral vectors carrying the indicated transgenes; Ad5-based vectors were used for prime immunization, and Ad5F35 vectors were used for boost immunization. Three weeks after boost, mice were challenged with 5,000 spleen focus-forming units (SFFU) of FV complex. Viral load in plasma (A) was analyzed 10 days after FV challenge; viral load in spleen (B) was analyzed on day 21 after FV challenge. Solid lines indicate median values. Statistically significant differences are indicated by asterisks (P < 0.05; ANOVA on ranks, Dunn's test). Dashed lines indicate the detection limit. Data were acquired in three independent experiments.