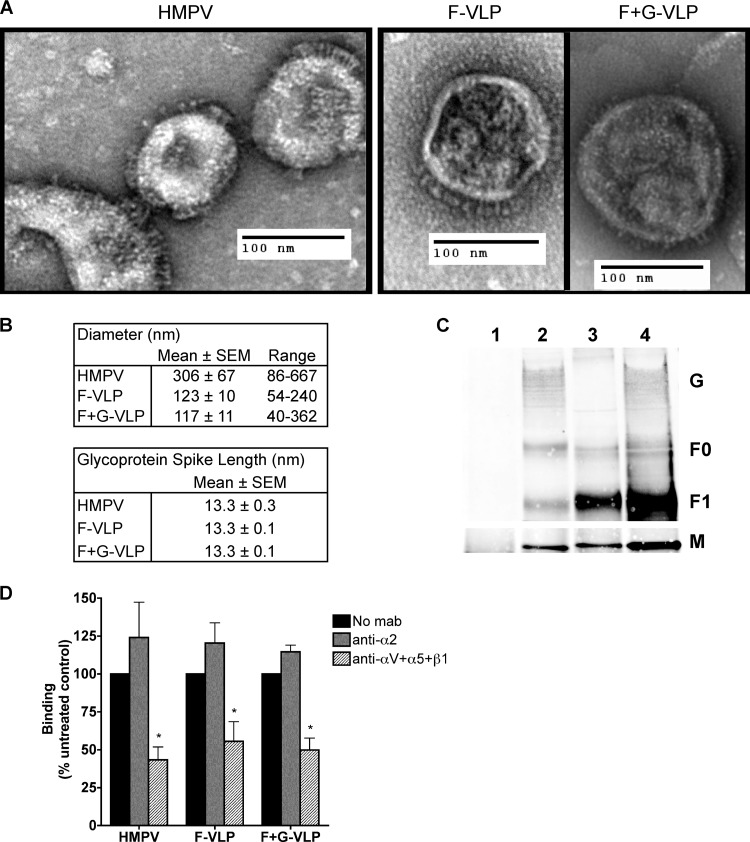
Fig 5.
HMPV F binds to RGD-binding integrins in the absence of G. (A) Electron micrographs of HMPV and F-only and F+G virus-like particles (VLPs). Sucrose-purified particles were stained with uranyl formate and imaged on an FEI Morgagni electron microscope (magnification, ×28,000). Protein spikes are visible projecting from the surfaces of both virus and VLPs. (B) Ten to 30 particles were chosen for morphology analysis; diameters and glycoprotein spike lengths were measured using AMT Image Capture Engine software. (C) Viral matrix (M), fusion (F), and attachment (G) protein incorporation into VLPs was confirmed by Western blotting. Virus and VLPs were immunoprecipitated with an anti-F monoclonal antibody (2 μg) and analyzed by Western blotting. Viral proteins were detected with HMPV M-specific or HMPV polyclonal A2 virus-specific (F and G) antibodies and fluorescent secondary antibodies using the Li-Cor Odyssey infrared imaging system. Lanes: 1, mock; 2, HMPV; 3, F-VLP; 4, F+G-VLP. Uncleaved protein (F0) and the large subunit from the cleaved form (F1) of the fusion protein were detected. M bands were detected in a different channel than F and G bands on the same blot and thus appear on a different image. (D) R18-MPV or R18-VLP binding was measured in the absence or presence of integrin function-blocking antibodies (α2 or a combination of αV, α5, and β1 to block all available RGD-binding integrins). Results from three independent experiments are expressed as mean percent inhibition relative to an untreated control (no MAb). Error bars indicate SEM; *, P ≤ 0.05.