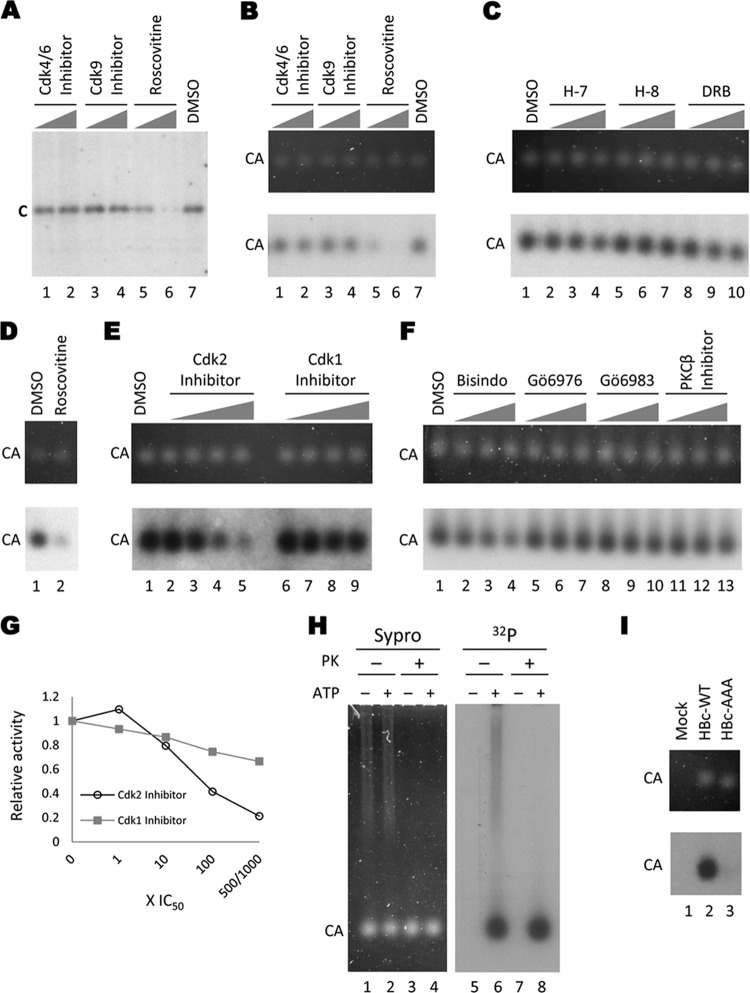
Fig 1.
Endogenous kinase reactions with HepG2-derived HBV capsids. HBV capsids isolated from HepG2 cells transfected with pCMV-HBV (all lanes except in panels D and I) or pCMV-HBV/pol− (D) were phosphorylated by the encapsidated kinase in the in vitro endogenous kinase assay in the presence of the indicated kinase inhibitors. The reaction products were resolved by SDS-PAGE to visualize the total core protein (after disruption of the capsids by SDS) (A) or by agarose gel electrophoresis to visualize capsid particles (B to F). Core proteins were detected by autoradiography (A; B to F, bottom) or Sypro ruby staining (B to F, top). Kinase inhibitors used were CDK4/6 inhibitor (A and B [lane 1, 15 μM; lane 2, 150 μM]), CDK9 inhibitor (A and B [lane 3, 350 nM; lane 4, 3.5 μM]), roscovitine (A and B [lane 5, 10 μM; lane 6, 100 μM] and D [lane 2, 100 μM]), H-7 (C [lane 2, 37 μM; lane 3, 370 μM; lane 4, 750 μM]), H-8 (C [lane 5, 62 μM; lane 6, 470 μM; lane 7, 590 μM]), DRB (C [lane 8, 30 μM; lane 9, 200 μM; lane 10, 300 μM]), CDK2 inhibitor (E [lane 2, 0.5 μM; lane 3, 5 μM; lane 4, 50 μM; lane 5, 250 μM]), CDK1 inhibitor (E [lane 6, 35 nM; lane 7, 350 nM; lane 8, 3.5 μM; lane 9, 35 μM]), Bisindo (F [lane 2, 100 nM; lane 3, 1 μM; lane 4, 5 μM]), Gö6976 (F [lane 5, 62 nM; lane 6, 620 nM; lane 7, 3.1 μM]), Gö6983 (F [lane 8, 70 nM; lane 9, 700 nM; lane 10, 3.5 μM]), PKCβ inhibitor (F [lane 11, 210 nM; lane 12, 2.1 μM; lane 13, 10.5 μM]), or DMSO control (A and B [lane 7] and C to F [lane 1]). The graph in panel G shows the endogenous kinase activity in the presence of CDK2 inhibitor or CDK1 inhibitor at increasing concentrations relative to the DMSO control. Open circles, CDK2 inhibitor; filled squares, CDK1 inhibitor. (H) Protease protection. Sucrose gradient-purified HBV capsids from HepG2 cells were treated with proteinase K (lanes 3 and 4 and 7 and 8) or mock treated (lanes 1 and 2 and 5 and 6) and subjected to an endogenous kinase reaction (even-number lanes) or a control reaction with no ATP (odd-number lanes). The samples were resolved on an agarose gel and visualized by Sypro ruby staining (left panel) or autoradiography (right panel). (I) Endogenous kinase reactions with WT and mutant HBV capsids. WT (lane 2) or the phosphorylation site mutant (AAA) (lane 3) HBV capsids were harvested from transiently transfected HepG2 cells by PEG precipitation. The crude capsids were then treated with proteinase K before being subjected to the endogenous kinase reaction. A sample from mock-transfected cells was also included (lane 1). The reactions were resolved on an agarose gel; capsids were visualized by Sypro ruby staining (top panel), and labeling by the endogenous kinase was detected by autoradiography (bottom panel). C, core protein; CA, capsids; PK, proteinase K.