Fig 5.
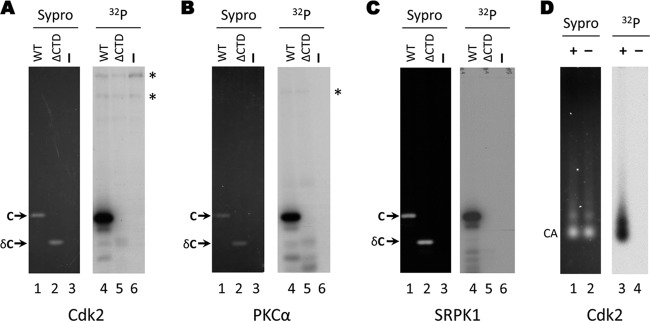
Phosphorylation of E. coli-derived HBV capsids by purified kinases in vitro. Exogenous kinase assays using CDK2-cyclin E1 (A), PKCα (B), or SRPK1 (C) were conducted with recombinant HBV capsids purified from bacteria. The products were resolved by SDS-PAGE (lanes 1 to 6) and visualized by autoradiography (lanes 1 to 3) or Sypro ruby staining (lanes 4 to 6). Lanes 1 and 4, full-length E. coli-derived capsid (WT; 1 to 183); lanes 2 and 5, E. coli-derived truncated capsid lacking the CTD phospho domain (ΔCTD; 1 to 149). Lanes 3 and 6, kinase assay containing no added substrate. (D) Exogenous CDK2-cyclin E1 kinase assays were conducted with E. coli-derived HBV capsids. The reaction products were resolved by agarose gel electrophoresis and visualized by Sypro ruby staining (lanes 1 and 2) or autoradiography (lanes 3 and 4). C, core protein; δC, ΔCTD, truncated core protein, 1 to 149; CA, capsids; *, background bands originating from the kinase preparation.
