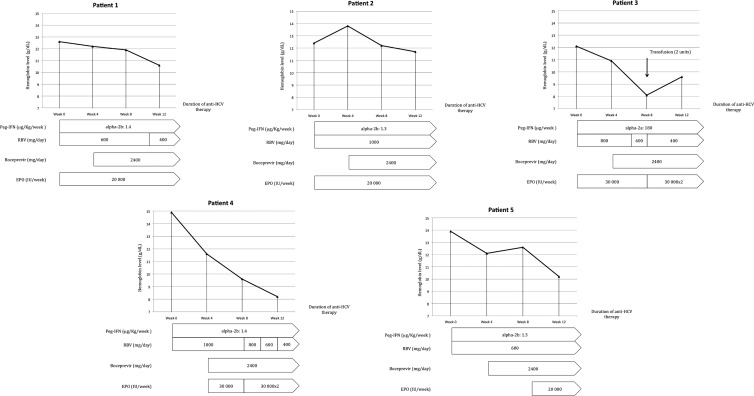
Fig 3.
Kinetics of hemoglobin levels during anti-HCV therapy and management. At baseline and week 12, the mean hemoglobin levels were 13.18 ± 1.18 g/dl (range, 12.1–14.9) and 10.4 ± 1.5 g/dl (range, 8.1 to 12.5), respectively. The mean fall in hemoglobin levels between baseline and week 12 was 3.12 ± 2.27 g/dl (range, 0.7 to 6.7). β-Erythropoietin was introduced in all patients if the hemoglobin level fell below 12 g/dl or if the hemoglobin level decreased by >1 g/dl/week and if a transfusion had been required during a previous anti-HCV therapy. In a second phase, the ribavirin dosage was reduced by between 33% and 75% in patients 1, 3, and 4. Only one patient required a transfusion because of a fall in the hemoglobin level to below 8 g/dl. In patients 2 and 5, MMF was maintained at the same dosage without a reduction of ribavirin. EPO, β-erythropoietin; RBV, ribavirin; Peg-IFN, pegylated interferon.