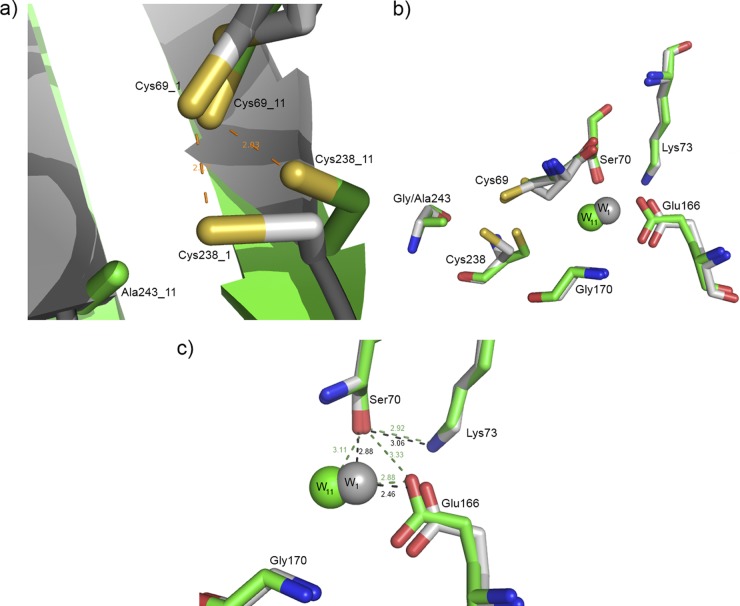
Fig 2.
(a) Conformation of the Cys238 residue is slightly modified in GES-11 (Cys238_11) compared to GES-1 (Cys238_1). (b) Superposition of the active sites of GES-11 (in green) and GES-1 (in gray). The hydrolytic water molecule and the Glu166 general base are slightly displaced. (c) The distances between Ser70 and the hydrolytic water molecule and between Glu166 and the hydrolytic water molecule are slightly larger in GES-11 than in GES-1. In GES-11, Glu166 forms an additional hydrogen bond with Ser70.