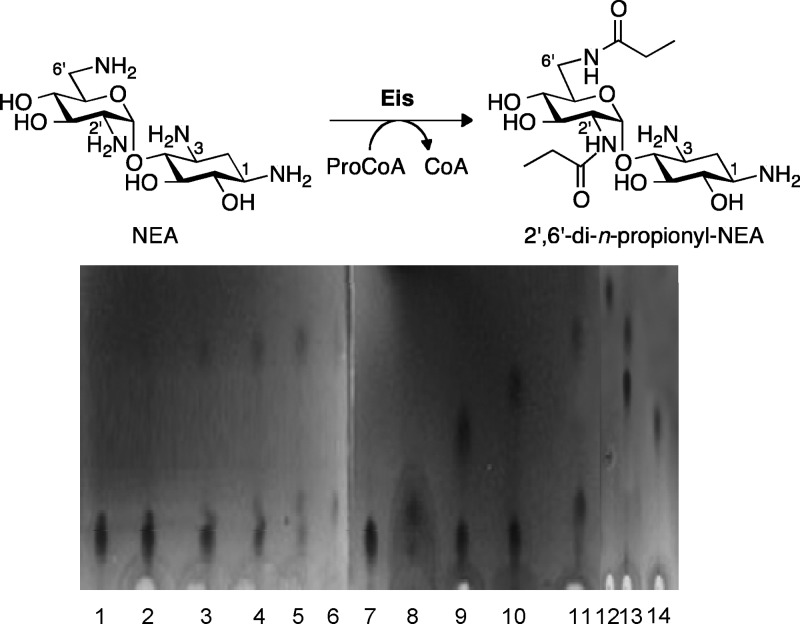
Fig 2.
TLC time course showing the 2′-mono- and 2′,6′-di-n-propionylated NEA products generated by Eis using 5 equivalents of ProCoA. Control reactions for mono- and di-n-propionylation were done using AAC(2′)-Ic, AAC(3)-IV, and AAC(6′) individually or sequentially. Lane 1, NEA (Rf 0.11); lane 2, NEA + ProCoA + Eis (1 min) (Rf 0.11, 0.13); lane 3, NEA + ProCoA + Eis (5 min) (Rf 0.11, 0.13, 0.41); lane 4, NEA + ProCoA + Eis (10 min) (Rf 0.11, 0.13, 0.41); lane 5, NEA + ProCoA + Eis (30 min) (Rf 0.11, 0.13. 0.41); lane 6, NEA + ProCoA + Eis (2 h) (Rf 0.11, 0.13. 0.41); lane 7, NEA (Rf 0.11); lane 8, 2′-n-propionyl-NEA (Rf 0.13); lane 9, 3-n-propionyl-NEA (Rf 0.24); lane 10, 6′-n-propionyl-NEA (Rf 0.33); lane 11, NEA + ProCoA + Eis (O/N) (Rf 0.41); lane 12, 6′,3-di-n-propionyl-NEA (Rf 0.53); lane 13, 6′,2′-di-n-propionyl-NEA (Rf 0.41); lane 14, 3,2′-di-n-propionyl-NEA (Rf 0.26).