Fig 4.
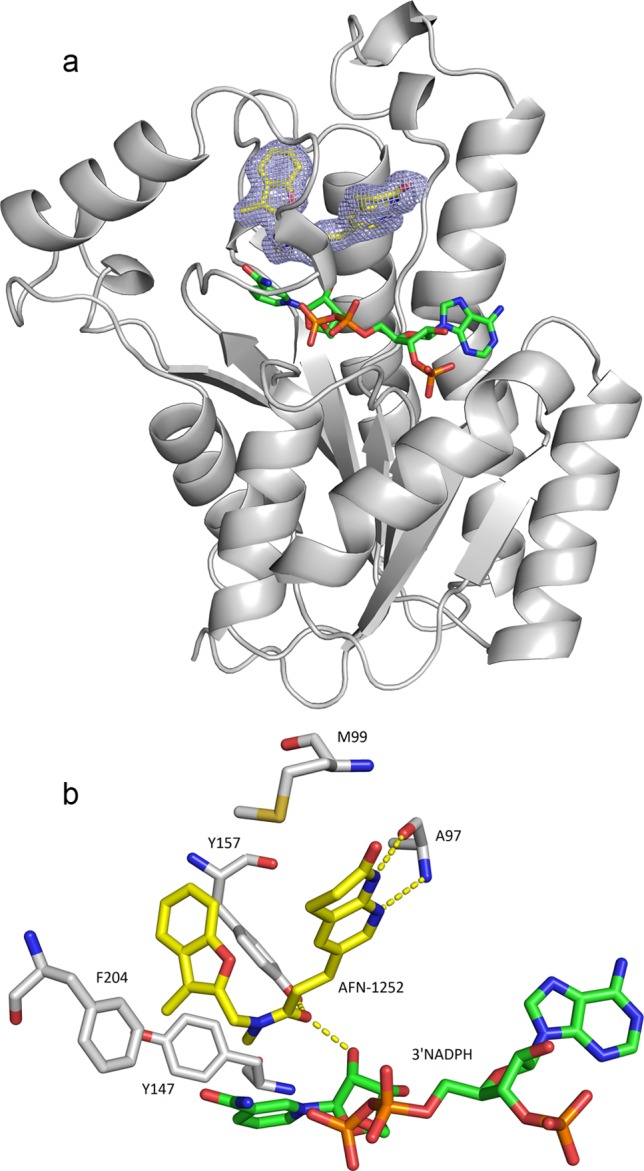
Cocrystal structure of AFN-1252–S. aureus FabI complex and schematic of key interactions. (a) Ribbon representation of the FabI structure with AFN-1252 and 3′NADPH in the binding pocket. Sigma A-weighted 2Fo − Fc electron density is shown for AFN-1252 in the region of the catalytic site at 1.8 Å resolution. The 3-methylbenzofuran side of AFN-1252 nestles in the hydrophobic pocket of the catalytic site, while the oxotetrahydronaphthyridine part of the molecule is more solvent exposed. AFN-1252 and 3′NADPH are shown as sticks, with carbon in yellow (AFN-1252) or green (3′NADPH), nitrogen in blue, oxygen in red, and sulfur in green. (b) Residues of FabI interacting with AFN-1252 and 3′NADPH. The hydroxyl of the ribose ring of 3′NADPH, A97, and Y157 form hydrogen bonds with the inhibitor, while M99, Y147, and F204 form a hydrophobic pocket. These images were generated with PyMOL (16).
