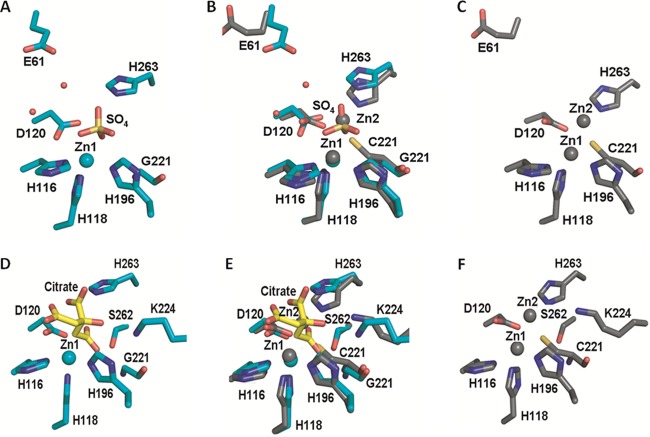
Fig 6.
Active-site residues of wild-type and C221G IMP-1 enzymes. (A, C, D, and F) Illustrations of wild-type (C and F) and C221G (sulfate-bound [A] and citrate-bound [D]) active-site residue conformations. (B and E) Overlays of the wild-type and C221G structures (sulfate-bound mutant [B] and citrate-bound mutant [E]). Residues are represented as sticks, with carbon atoms shown in gray (wild type) or teal (C221G mutant). Citrate carbon atoms are shown in yellow. Zinc ions are shown as gray (wild-type) or teal (C221G mutant) spheres, and water is shown as small red spheres. Nitrogen is shown in blue, sulfur in yellow, and oxygen in red. The figures were rendered using the PyMOL Molecular Graphics System (11).