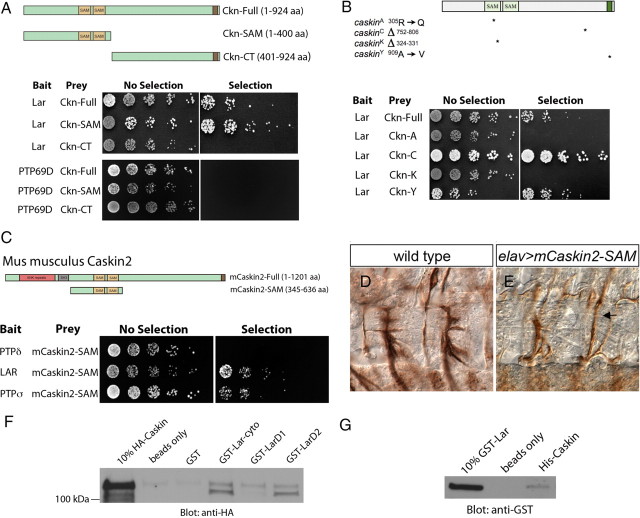
Figure 5.
Ckn and Lar interact physically. A, Yeast two-hybrid interactions indicate a direct physical interaction between Ckn and Lar, but not Ckn and PTP69D. The cytoplasmic domain of Lar was fused to the Gal4-DNA binding domain (bait). Schematics of the Ckn constructs that were fused to the Gal4-activation domain (prey) are shown. Both full-length Ckn and Ckn-SAM interact with Lar-cyto. In contrast, none of the Ckn constructs interacts with PTP69D. B, Schematics of four caskin mutant variants are shown. These mutant cDNAs were fused to the Gal4-activation domain (prey) and tested for binding with the Lar-cyto domain fused to the Gal4-DNA binding domain (bait). Both of the SAM domain mutations, Ckn-A and Ckn-K, abrogate Lar binding, whereas both Ckn-C and Ckn-Y maintain Lar interactions. C, The cytoplasmic domains of the three vertebrate type IIA RPTPs were fused to the Gal4-DNA binding domain (bait) and tested against full-length mCaskin2 and a fragment of mCaskin2 containing both SAM domains fused to the Gal4-activiation domain (prey). Lar and PTPσ interact with mCaskin-SAM. D, E, Two hemisegments of stage 16 wild-type (D) and elavGAL4/UAS-mCaskin2-SAM (E) embryos labeled with mAb 1D4. Neuronal overexpression of mCaskin2-SAM yields ISNb bypass (arrow) suggesting that it interferes with Lar signal transduction in a dominant-negative fashion. F, A GST-Lar-cyto fusion protein precipitates HA-Caskin from elavGAL4/UAS-HA-Caskin embryonic lysates, whereas GST alone does not. GST-Lar D2 pulls HA-Caskin out of embryonic lysates arguing that Ckn binds the D2 domain. G, A bacterially expressed and purified HA-Caskin fusion protein precipitates purified GST-Lar, whereas Ni-NTA beads alone cannot.